Allosteric Coupling of Drug Binding and Intracellular Signaling in the A2A Adenosine Receptor
- PMID: 29290469
- PMCID: PMC5766378
- DOI: 10.1016/j.cell.2017.12.004
Allosteric Coupling of Drug Binding and Intracellular Signaling in the A2A Adenosine Receptor
Abstract
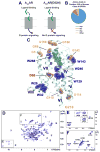
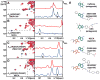
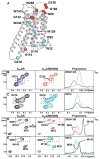
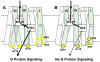
Signaling across cellular membranes, the 826 human G protein-coupled receptors (GPCRs) govern a wide range of vital physiological processes, making GPCRs prominent drug targets. X-ray crystallography provided GPCR molecular architectures, which also revealed the need for additional structural dynamics data to support drug development. Here, nuclear magnetic resonance (NMR) spectroscopy with the wild-type-like A2A adenosine receptor (A2AAR) in solution provides a comprehensive characterization of signaling-related structural dynamics. All six tryptophan indole and eight glycine backbone 15N-1H NMR signals in A2AAR were individually assigned. These NMR probes provided insight into the role of Asp522.50 as an allosteric link between the orthosteric drug binding site and the intracellular signaling surface, revealing strong interactions with the toggle switch Trp 2466.48, and delineated the structural response to variable efficacy of bound drugs across A2AAR. The present data support GPCR signaling based on dynamic interactions between two semi-independent subdomains connected by an allosteric switch at Asp522.50.
Keywords: G protein-coupled receptor; GPCR; NMR; allosteric modulation; membrane protein; nuclear magnetic resonance; signaling.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Audet M, Bouvier M. Restructuring G-Protein-Coupled Receptor Activation. Cell. 2012;151:14–23. - PubMed
-
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosi. 1995;25:366–428.
-
- Bara-Jimenez W, Sherzai A, Dimitrova T, Favit A, Bibbiani F, Gillespie M, Morris M, Mouradian M, Chase T. Adenosine A2A receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61:293–296. - PubMed
-
- Beck M, Sakmar TP, Siebert F. Spectroscopic evidence for interaction between transmembrane helices 3 and 5 in rhodopsin. Biochemistry. 1998;37:7630–7639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

