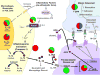
Figure 2
Genetic Associations to EARR within the ATP/P2RX7/IL-1β and RANK/RANKL/OPN signaling pathways-The pie-charts located throughout the diagram summarize the genetic association and linkage findings connected to different factors within the pathways and EARR. The number of triangles within each pie shape summarize the number of markers or independent tests that were examined for each factor; where (green) indicates genetic association, (red) indicates no association, (yellow) indicates a haplotype association, and (blue) indicates genetic linkage. As orthodontic force is placed on the teeth and the neighboring periodontal ligament (PDL) is compressed, the immune system responds to the site in order to relieve the tissue stress and damage. As part of the stress response, ATP is released from platelets and binds to the purinergic receptor P2X, ligand-gated ion channel 7 (P2RX7) membrane channel protein located on the surface of immune cells and/or cells of the PDL. Upon binding ATP, the P2RX7 ion channel is opened, allowing the exchange of intracellular potassium (K+) and extracellular sodium (Na++), and triggering the elevation of calcium (Ca++) from intracellular stores. Elevation of intracellular Ca++ activates caspase-1 (also termed IL-1β converting enzyme or ICE) which is located in inflammasome complexes within the cell. Caspase-1 cleaves the pro-IL-1β molecule, releasing active mature interleukin-1beta (IL-1β) for biological function. IL-1β can recruit other inflammatory cells to the site of tissue damage, and can bind to interleukin-1 receptors (IL-1R) on the surface of pro-osteoblastic cells. The IL-1 receptor antagonist (IL1RA) may interrupt IL-1 signaling. Once IL-1β is bound to the IL-1Rs, a signaling cascade involving the Interleukin-1 Receptor Associated Kinase 1 (IRAK1) and other molecules (not shown) lead to the activation of such genes as the receptor activator of nuclear factor kappa-B ligand (RANKL) and Osteoprotegerin (OPG). When RANKL is synthesized, and expressed on the surface of the osteoblastic cells it can bind RANK on preosteoclasts. This interaction, in concert with M-CSF production from osteoblasts and binding to the c-fms receptor on the surface of pre-osteoclastic cells signals the osteoclast precursor cells to mature into functional osteoclasts. OPG and soluble RANKL (sRANKL) can act to dampen the maturation signal to pro-osteoclast cells by interfering with RANKL:RANK interactions. Osteopontin (OPN) plays an important role in bone remodeling by increasing osteoclast anchoring. The action of both osteoblasts and osteoclasts are needed to resolve the tissue stresses within the PDL from orthodontic force application. Additional associations with EARR that not depicted in this figure include the vitamin D receptor (VDR) on osteoblasts and Interleukin-6 (IL-6). IL-1a (Al-Qawasmi, et.al., 2003) Am J Orthod Dentofacial Orthop 123:242–52 (Gülden, et al., 2009) J Orofac Orthop 70: 20–18 (Iglesias-Linares, et. al., 2012) Oral Diseases 18:198–205 (Iglesias-Linares, et. al., 2012) International Endodontic Journal 45: 1018–1026 (Iglesias-Linares, et. al., 2012) Journal of Endodontics, 2012, Vol.38(3), p.283–287 (Linhartova, et. al., 2013) Oral Diseases 19:262–70 (Sharab, et al., 2015) Orthod Craniofac Res. 2015 Apr;18 Suppl 1:71–82. doi: 10.1111/ocr.12078 IL-1β (Al-Qawasmi, et al., 2003) Am J Orthod Dentofacial Orthop 123:242–52 (Bastos Lages, et al., 2009) Am J Orthod Dentofacial Orthop 136:542–6 (Gülden, et al., 2009) J Orofac Orthop 70: 20–18 (Iglesias-Linares, et al., 2012) Oral Diseases 18:198–205 (Iglesias-Linares, 2012) International Endodontic Journal 45: 1018–1026 (Iglesias-Linares, et. al., 2012) Journal of Endodontics,38: 283–287 (Linhartova, et. al., 2013) Oral Diseases 19:262–70 Pereira, et. al., 2014) Oral Dis 20: 659–667 (Pereira, et. al., 2016) Oral Diseases (2016) doi: 10.1111/odi.12514 (Sharab, et al., 2015) Orthod Craniofac Res. 2015 Apr;18 Suppl 1:71–82. doi: 10.1111/ocr.12078. IL-1RA Pereira, et. al., 2016) Oral Diseases (2016) doi: 10.1111/odi.12514 (Iglesias-Linares, et. al., 2012) Oral Diseases 18:198–205 (Iglesias-Linares, et. al., 2013) Histol Histopathol. 28:767–73. (Sharab, et al., 2015) Orthod Craniofac Res. 2015 Apr;18 Suppl 1:71–82. doi: 10.1111/ocr.12078 (Linhartova, et. al., 2013) Oral Diseases 19:262–70 (Gua, et al., 2016) Am J Orthod Dentofacial Orthop. 150:283–9 IRAK1 (Pereira, et. al., 2016) Oral Diseases (2016) doi: 10.1111/odi.12514 Caspase-1 (Sharab, et al., 2015) Orthod Craniofac Res. 2015 Apr;18 Suppl 1:71–82. doi:10.1111/ocr.12078. → (3 different SNPs examined) P2RX7 (Sharab, et al., 2015) Orthod Craniofac Res. 2015 Apr;18 Suppl 1:71–82. doi:10.1111/ocr.12078. → 3 different SNPs examined (Linhartova, et. al., 2016) Oral Dis. 2016 Aug 5. doi: 10.1111/odi.12564. [Epub ahead of print] (Pereira, et. al., 2014) Oral Dis 20: 659–667 (Linhartova, et. l., 2016) Oral Dis. 2016 Aug 5. doi: 10.1111/odi.12564. [Epub ahead of print] RANK (Al-Qawasmi, et. al., 2003) J Dent Res. 2003 May;82(5):356–60 (Pereira, et. al., 2014) Oral Dis 20: 659–667 OPG (Hartsfield, 2009) Orthod Craniofac Res 12: 236–242 (Pereira, et. al., 2014) Oral Dis 20: 659–667 (Linhartova, et. al., 2016) Oral Dis. 2016 Aug 5. doi: 10.1111/odi.12564. [Epub ahead of print] → 2 SNPs examined OPN (Iglesias-Linares, et. al., 2014) Oral Diseases 20: 307–312 (Linhartova, et. al., 2016) Oral Dis. 2016 Aug 5. doi: 10.1111/odi.12564. [Epub ahead of print] → 2 SNPs examined (Iglesias-Linares, 2014) Oral Diseases 20: 307–312 VDR (Fontana, et al., 2012) Am J Orthod Dentofacial Orthop. 142:339–47 IL-6 (Gua, et al., 2016) Am J Orthod Dentofacial Orthop. 150:283–9