Histidine-rich Modification of a Scorpion-derived Peptide Improves Bioavailability and Inhibitory Activity against HSV-1
- PMID: 29290802
- PMCID: PMC5743469
- DOI: 10.7150/thno.21425
Histidine-rich Modification of a Scorpion-derived Peptide Improves Bioavailability and Inhibitory Activity against HSV-1
Abstract
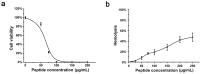
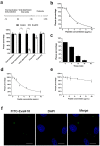
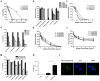
Rationale: HSV is one of the most widespread human viral pathogens. HSV-1 infects a large portion of the human population and causes severe diseases. The current clinical treatment for HSV-1 is based on nucleoside analogues, the use of which is limited due to drug resistance, side effects and poor bioavailability. AMPs have been identified as potential antiviral agents that may overcome these limitations. Therefore, we screened anti-HSV-1 peptides from a scorpion-derived AMP library and engineered one candidate into a histidine-rich peptide with significantly improved antiviral activity and development potential. Methods: A venomous gland cDNA library was constructed from the scorpion Euscorpiops validus in the Yunnan Province of China. Six putative AMPs were characterized from this cDNA library, and the synthesized peptides were screened via plaque-forming assays to determine their virucidal potential. Time of addition experiments according to the infection progress of HSV-1 were used to identify the modes of action for peptides of interest. The histidine-rich modification was designed based on structural analysis of peptides by a helical wheel model and CD spectroscopy. Peptide cellular uptake and distribution were measured by flow cytometry and confocal microscopy, respectively. Results: The peptide Eval418 was found to have high clearance activity in an HSV-1 plaque reduction assay. Eval418 exhibited dose-dependent and time-dependent inactivation of HSV-1 and dose-dependent inhibition of HSV-1 attachment to host cells. However, Eval418 scarcely suppressed an established HSV-1 infection due to poor cellular uptake. We further designed and modified Eval418 into four histidine-rich derivative peptides with enhanced antiviral activities and lower cytotoxicities. All of the derivative peptides suppressed established HSV-1 infections. One of these peptides, Eval418-FH5, not only had strong viral inactivation activity and enhanced attachment inhibitory activity but also had high inhibitory activity against intracellular HSV-1, which was consistent with its improved intracellular uptake and distribution as confirmed by confocal microscopy and flow cytometry. Conclusion: We successfully identified an anti-HSV-1 peptide, Eval418, from a scorpion venom peptide library and designed a histidine-rich Eval418 derivative with significantly improved potential for further development as an anti-HSV-1 drug. This successful modification can provide a design strategy to improve the bioavailability, cellular distribution and antiviral activity of peptide agents.
Keywords: Anti-HSV-1 peptides; Bioavailability.; Cellular uptake; Histidine-rich modification; Scorpion venom peptides.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Brandt C R. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Experimental eye research. 2005;80:607–21. - PubMed
-
- Liashkovich I, Hafezi W, Kuhn J M, Oberleithner H, Shahin V. Nuclear delivery mechanism of herpes simplex virus type 1 genome. Journal of molecular recognition: JMR. 2011;24:414–21. - PubMed
-
- Schacker T. The role of HSV in the transmission and progression of HIV. Herpes: the journal of the IHMF. 2001;8:46–9. - PubMed
-
- Schang L M. Herpes simplex viruses in antiviral drug discovery. Current pharmaceutical design. 2006;12:1357–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

