CD4 Lymphocyte Enumeration and Hemoglobin Assessment Aid for Priority Decisions: A Multisite Evaluation of the BD FACSPresto™ System
- PMID: 29290885
- PMCID: PMC5730955
- DOI: 10.2174/1874613601711010076
CD4 Lymphocyte Enumeration and Hemoglobin Assessment Aid for Priority Decisions: A Multisite Evaluation of the BD FACSPresto™ System
Abstract
Background: The BD FACSPresto™ system uses capillary and venous blood to measure CD4 absolute counts (CD4), %CD4 in lymphocytes, and hemoglobin (Hb) in approximately 25 minutes. CD4 cell count is used with portable CD4 counters in resource-limited settings to manage HIV/AIDS patients. A method comparison was performed using capillary and venous samples from seven clinical laboratories in five countries. The BD FACSPresto system was assessed for variability between laboratory, instrument/operators, cartridge lots and within-run at four sites.
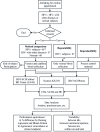
Methods: Samples were collected under approved voluntary consent. EDTA-anticoagulated venous samples were tested for CD4 and %CD4 T cells using the gold-standard BD FACSCalibur™ system, and for Hb, using the Sysmex® KX-21N™ analyzer. Venous and capillary samples were tested on the BD FACSPresto system. Matched data was analyzed for bias (Deming linear regression and Bland-Altman methods), and for concordance around the clinical decision point. The coefficient of variation was estimated per site, instrument/operator, cartridge-lot and between-runs.
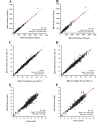
Results: For method comparison, 93% of the 720 samples were from HIV-positive and 7% from HIV-negative or normal subjects. CD4 and %CD4 T cells venous and capillary results gave slopes within 0.96-1.05 and R2 ≥0.96; Hb slopes were ≥1.00 and R2 ≥0.89. Variability across sites/operators gave %CV <5.8% for CD4 counts, <1.9% for %CD4 and <3.2% for Hb. The total %CV was <7.7% across instrument/cartridge lot.
Conclusion: The BD FACSPresto system provides accurate, reliable, precise CD4/%CD4/Hb results compared to gold-standard methods, irrespective of venous or capillary blood sampling. The data showed good agreement between the BD FACSPresto, BD FACSCalibur and Sysmex systems.
Keywords: CD4; Capillary; HIV-1 diversity; HIV/AIDS; Hemoglobin; Precision; Recent infections; Venous.
Figures

References
-
- WHO, UNAIDS, UNICEF, Global HIV/AIDS response. Epidemic update and health sector progress towards Universal Access. Progress Report 2011. Progress Report [Internet]. Available from: http://apps.who.int/iris/bitstream/ 10665/44787/1/9789241502986_eng.pdf . 2011. 2017 Jan 10.
-
- Center for Disease Control and Prevention. Revised Guidelines for Performning CD4+ T-Cell Determinations in Persons Infected with Human Immunodeficiency Virus (HIV). MMWR 1997;46(No. RR2) 10 January. - PubMed
-
- Shearer W.T., Rosenblatt H.M., Gelman R.S., Oyomopito R., Plaeger S., Stiehm E.R., Wara D.W., Douglas S.D., Luzuriaga K., McFarland E.J., Yogev R., Rathore M.H., Levy W., Graham B.L., Spector S.A., Pediatric AIDS Clinical Trials Group Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J. Allergy Clin. Immunol. 2003;112(5):973–980. doi: 10.1016/j.jaci.2003.07.003. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
