Glucose-6-phosphate dehydrogenase and transketolase modulate breast cancer cell metabolic reprogramming and correlate with poor patient outcome
- PMID: 29290982
- PMCID: PMC5739767
- DOI: 10.18632/oncotarget.21601
Glucose-6-phosphate dehydrogenase and transketolase modulate breast cancer cell metabolic reprogramming and correlate with poor patient outcome
Abstract
The pentose phosphate pathway is a fundamental metabolic pathway that provides cells with ribose and NADPH required for anabolic reactions - synthesis of nucleotides and fatty acids - and maintenance of intracellular redox homeostasis. It plays a key role in tumor metabolic reprogramming and has been reported to be deregulated in different types of tumors. Herein, we silenced the most important enzymes of this pathway - glucose-6-phosphate dehydrogenase (G6PD) and transketolase (TKT) - in the human breast cancer cell line MCF7. We demonstrated that inhibition of G6PD, the oxidative branch-controlling enzyme, reduced proliferation, cell survival and increased oxidative stress. At the metabolic level, silencing of both enzymes reduced ribose synthesis. G6PD silencing in particular, augmented the glycolytic flux, reduced lipid synthesis and increased glutamine uptake, whereas silencing of TKT reduced the glycolytic flux. Importantly, we showed using breast cancer patient datasets that expression of both enzymes is positively correlated and that high expression levels of G6PD and TKT are associated with decreased overall and relapse-free survival. Altogether, our results suggest that this metabolic pathway could be subjected to therapeutic intervention to treat breast tumors and warrant further investigation.
Keywords: breast cancer; glucose-6-phosphate dehydrogenase; pentose phosphate pathway; transketolase; tumor metabolism.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no competing interests.
Figures

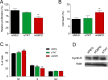


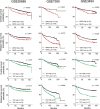
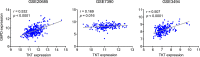
References
-
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–82. - PubMed
-
- Tarrado-Castellarnau M, de Atauri P, Cascante M. Oncogenic regulation of tumor metabolic reprogramming. Oncotarget. 2016;7:62726–53. https://doi.org/10.18632/oncotarget.10911. - DOI - PMC - PubMed
-
- Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53:421–36. - PubMed
-
- Kuehne A, Emmert H, Soehle J, Winnefeld M, Fischer F, Wenck H, Gallinat S, Terstegen L, Lucius R, Hildebrand J, Zamboni N. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol Cell. 2015;59:359–71. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

