Catalytic α-Arylation of Imines Leading to N-Unprotected Indoles and Azaindoles
- PMID: 29291137
- PMCID: PMC5745074
- DOI: 10.1021/acscatal.6b00040
Catalytic α-Arylation of Imines Leading to N-Unprotected Indoles and Azaindoles
Abstract
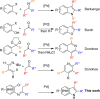
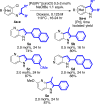
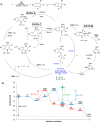
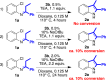
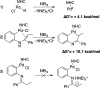
A palladium N-heterocyclic carbene catalyzed methodology for the synthesis of substituted, N-unprotected indoles and azaindoles is reported. The protocol permits access to various, highly substituted members of these classes of compounds. Although two possible reaction pathways (deprotonative and Heck-like) can be proposed, control experiments, supported by computational studies, point toward a deprotonative mechanism being operative.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Horton D. A.; Bourne G. T.; Smythe M. L. Chem. Rev. 2003, 103, 893–930. 10.1021/cr020033s. - DOI - PubMed
- Galliford C. V.; Scheidt K. A. Angew. Chem., Int. Ed. 2007, 46, 8748–8758. 10.1002/anie.200701342. - DOI - PubMed
- O’Hagan D. Nat. Prod. Rep. 2000, 17, 435–446. 10.1039/a707613d. - DOI - PubMed
- Michael J. P. Nat. Prod. Rep. 2005, 22, 627–646. 10.1039/b413750g. - DOI - PubMed
-
- Hughes G.; Bryce M. R. J. Mater. Chem. 2005, 15, 94–107. 10.1039/b413249c. - DOI
-
- Hegedus L. S.; Mulhern T. A.; Mori A. J. Org. Chem. 1985, 50, 4282–4288. 10.1021/jo00222a017. - DOI
-
- Larock R. C.; Yum E. K. J. Am. Chem. Soc. 1991, 113, 6689–6690. 10.1021/ja00017a059. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
