Developmental Emergence of Phenotypes in the Auditory Brainstem Nuclei of Fmr1 Knockout Mice
- PMID: 29291238
- PMCID: PMC5744645
- DOI: 10.1523/ENEURO.0264-17.2017
Developmental Emergence of Phenotypes in the Auditory Brainstem Nuclei of Fmr1 Knockout Mice
Abstract
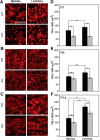
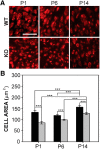
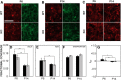
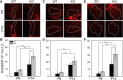
Fragile X syndrome (FXS), the most common monogenic cause of autism, is often associated with hypersensitivity to sound. Several studies have shown abnormalities in the auditory brainstem in FXS; however, the emergence of these auditory phenotypes during development has not been described. Here, we investigated the development of phenotypes in FXS model [Fmr1 knockout (KO)] mice in the ventral cochlear nucleus (VCN), medial nucleus of the trapezoid body (MNTB), and lateral superior olive (LSO). We studied features of the brainstem known to be altered in FXS or Fmr1 KO mice, including cell size and expression of markers for excitatory (VGLUT) and inhibitory (VGAT) synapses. We found that cell size was reduced in the nuclei with different time courses. VCN cell size is normal until after hearing onset, while MNTB and LSO show decreases earlier. VGAT expression was elevated relative to VGLUT in the Fmr1 KO mouse MNTB by P6, before hearing onset. Because glial cells influence development and are altered in FXS, we investigated their emergence in the developing Fmr1 KO brainstem. The number of microglia developed normally in all three nuclei in Fmr1 KO mice, but we found elevated numbers of astrocytes in Fmr1 KO in VCN and LSO at P14. The results indicate that some phenotypes are evident before spontaneous or auditory activity, while others emerge later, and suggest that Fmr1 acts at multiple sites and time points in auditory system development.
Keywords: auditory; brainstem; fragile x; lateral superior olive; medial nucleus of the trapezoid body; ventral cochlear nucleus:.
Conflict of interest statement
Authors report no conflict of interest.
Figures






Similar articles
-
Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem.PLoS One. 2015 Feb 13;10(2):e0117266. doi: 10.1371/journal.pone.0117266. eCollection 2015. PLoS One. 2015. PMID: 25679778 Free PMC article.
-
Enhanced Excitatory Connectivity and Disturbed Sound Processing in the Auditory Brainstem of Fragile X Mice.J Neurosci. 2017 Aug 2;37(31):7403-7419. doi: 10.1523/JNEUROSCI.2310-16.2017. Epub 2017 Jul 3. J Neurosci. 2017. PMID: 28674175 Free PMC article.
-
Abnormal neuronal morphology and neurochemistry in the auditory brainstem of Fmr1 knockout rats.Neuroscience. 2015 Sep 10;303:285-98. doi: 10.1016/j.neuroscience.2015.06.061. Epub 2015 Jul 9. Neuroscience. 2015. PMID: 26166728
-
Sensory Processing Phenotypes in Fragile X Syndrome.ASN Neuro. 2018 Jan-Dec;10:1759091418801092. doi: 10.1177/1759091418801092. ASN Neuro. 2018. PMID: 30231625 Free PMC article. Review.
-
The fragile X-cerebellum connection.Trends Neurosci. 2006 Apr;29(4):183-5. doi: 10.1016/j.tins.2006.02.001. Epub 2006 Feb 28. Trends Neurosci. 2006. PMID: 16500716 Review.
Cited by
-
CX3CR1 mutation alters synaptic and astrocytic protein expression, topographic gradients, and response latencies in the auditory brainstem.J Comp Neurol. 2021 Aug 1;529(11):3076-3097. doi: 10.1002/cne.25150. Epub 2021 Apr 14. J Comp Neurol. 2021. PMID: 33797066 Free PMC article.
-
Characterization of Auditory and Binaural Spatial Hearing in a Fragile X Syndrome Mouse Model.eNeuro. 2020 Jan 31;7(1):ENEURO.0300-19.2019. doi: 10.1523/ENEURO.0300-19.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31953317 Free PMC article.
-
Tonotopic differentiation of presynaptic neurotransmitter-releasing machinery in the auditory brainstem during the prehearing period and its selective deficits in Fmr1 knockout mice.J Comp Neurol. 2022 Dec;530(18):3248-3269. doi: 10.1002/cne.25406. Epub 2022 Sep 6. J Comp Neurol. 2022. PMID: 36067267 Free PMC article.
-
Abnormal development of auditory responses in the inferior colliculus of a mouse model of Fragile X Syndrome.J Neurophysiol. 2020 Jun 1;123(6):2101-2121. doi: 10.1152/jn.00706.2019. Epub 2020 Apr 22. J Neurophysiol. 2020. PMID: 32319849 Free PMC article.
-
Myelination Deficits in the Auditory Brainstem of a Mouse Model of Fragile X Syndrome.Front Neurosci. 2021 Nov 11;15:772943. doi: 10.3389/fnins.2021.772943. eCollection 2021. Front Neurosci. 2021. PMID: 34858133 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
