TFPIα interacts with FVa and FXa to inhibit prothrombinase during the initiation of coagulation
- PMID: 29291252
- PMCID: PMC5745139
- DOI: 10.1182/bloodadvances.2017011098
TFPIα interacts with FVa and FXa to inhibit prothrombinase during the initiation of coagulation
Abstract
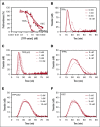
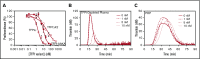
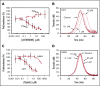
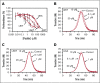
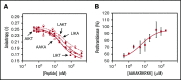
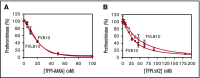
Tissue factor pathway inhibitor α (TFPIα) inhibits prothrombinase, the thrombin-generating complex of factor Xa (FXa) and factor Va (FVa), during the initiation of coagulation. This inhibition requires binding of a conserved basic region within TFPIα to a conserved acidic region in FXa-activated and platelet-released FVa. In this study, the contribution of interactions between TFPIα and the FXa active site and FVa heavy chain to prothrombinase inhibition were examined to further define the inhibitory biochemistry. Removal of FXa active site binding by mutation or by deletion of the second Kunitz domain (K2) of TFPIα produced 17- or 34-fold weaker prothrombinase inhibition, respectively, establishing that K2 binding to the FXa active site is required for efficient inhibition. Substitution of the TFPIα basic region uncharged residues (Leu252, Ile253, Thr255) with Ala (TFPI-AAKA) produced 5.8-fold decreased inhibition. This finding was confirmed using a basic region peptide (Leu252-Lys261) and Ala substitution peptides, which established that the uncharged residues are required for prothrombinase inhibitory activity but not for binding the FVa acidic region. This suggests that the uncharged residues mediate a secondary interaction with FVa subsequent to acidic region binding. This secondary interaction seems to be with the FVa heavy chain, because the FV Leiden mutation weakened prothrombinase inhibition by TFPIα but did not alter TFPI-AAKA inhibitory activity. Thus, efficient inhibition of prothrombinase by TFPIα requires at least 3 intermolecular interactions: (1) the TFPIα basic region binds the FVa acidic region, (2) K2 binds the FXa active site, and (3) Leu252-Thr255 binds the FVa heavy chain.
Conflict of interest statement
Conflict-of-interest disclosure: A.E.M. receives grant support from Novo Nordisk. H.H.P., B.Y., X.W., and I.H. are employees of Novo Nordisk A/S. J.P.W. declares no competing financial interests.
Figures


References
-
- Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76(1):1-16. - PubMed
-
- Miletich JP, Kane WH, Hofmann SL, Stanford N, Majerus PW. Deficiency of factor Xa-factor Va binding sites on the platelets of a patient with a bleeding disorder. Blood. 1979;54(5):1015-1022. - PubMed
-
- Zhu H, Toso R, Camire RM. Inhibitory sequences within the B-domain stabilize circulating factor V in an inactive state. J Biol Chem. 2007;282(20):15033-15039. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

