Shared mechanisms regulate spatiotemporal RhoA-dependent actomyosin contractility during adhesion and cell division
- PMID: 29291271
- PMCID: PMC7053959
- DOI: 10.1080/21541248.2017.1366966
Shared mechanisms regulate spatiotemporal RhoA-dependent actomyosin contractility during adhesion and cell division
Abstract
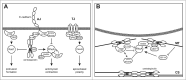
Local modulation of the actin cytoskeleton is essential for the initiation and maintenance of strong homotypic adhesive interfaces between neighboring cells. The epithelial adherens junction (AJ) fulfils a central role in this process by mediating E-cadherin interactions and functioning as a signaling scaffold to control the activity of the small GTPase RhoA and subsequent actomyosin contractility. Interestingly, a number of regulatory proteins that modulate RhoA activity at the AJ also control RhoA during cytokinesis, an actomyosin-dependent process that divides the cytoplasm to generate two daughter cells at the final stages of mitosis. Recent insights have revealed that the central player in AJ stability, p120-catenin (p120), interacts with and modulates essential regulators of actomyosin contraction during cytokinesis. In cancer, loss of this modulation is a common event during tumor progression that can induce chromosomal instability and tumor progression.In this review, we will highlight the functional differences and similarities of the different RhoA-associated factors that have been linked to both the regulation of cell-cell adhesion and cytokinesis.
Keywords: Rho activity; actomyosin contractility; cell adhesion; cytokinesis; p120-catenin.
Figures

Similar articles
-
p120-catenin prevents multinucleation through control of MKLP1-dependent RhoA activity during cytokinesis.Nat Commun. 2016 Dec 22;7:13874. doi: 10.1038/ncomms13874. Nat Commun. 2016. PMID: 28004812 Free PMC article.
-
Deficient E-cadherin adhesion in C57BL/6J-Min/+ mice is associated with increased tyrosine kinase activity and RhoA-dependent actomyosin contractility.Exp Cell Res. 2006 Feb 15;312(4):387-400. doi: 10.1016/j.yexcr.2005.11.019. Exp Cell Res. 2006. PMID: 16368433
-
Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells.Dev Cell. 2013 Feb 11;24(3):242-55. doi: 10.1016/j.devcel.2013.01.008. Dev Cell. 2013. PMID: 23410939
-
Beyond regulation of cell adhesion: local control of RhoA at the cleavage furrow by the p0071 catenin.Cell Cycle. 2007 Jan 15;6(2):122-7. doi: 10.4161/cc.6.2.3741. Epub 2007 Jan 19. Cell Cycle. 2007. PMID: 17264675 Review.
-
Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function.Exp Cell Res. 2017 Sep 1;358(1):20-30. doi: 10.1016/j.yexcr.2017.03.053. Epub 2017 Mar 29. Exp Cell Res. 2017. PMID: 28363828 Free PMC article. Review.
Cited by
-
ARHGEF17/TEM4 regulates the cell cycle through control of G1 progression.J Cell Biol. 2025 Mar 3;224(3):e202311194. doi: 10.1083/jcb.202311194. Epub 2025 Feb 4. J Cell Biol. 2025. PMID: 39903211
-
A Novel Single-Color FRET Sensor for Rho-Kinase Reveals Calcium-Dependent Activation of RhoA and ROCK.Sensors (Basel). 2024 Oct 26;24(21):6869. doi: 10.3390/s24216869. Sensors (Basel). 2024. PMID: 39517770 Free PMC article.
-
N-cadherin antagonism is bronchoprotective in severe asthma models.Sci Adv. 2024 Nov 29;10(48):eadp8872. doi: 10.1126/sciadv.adp8872. Epub 2024 Nov 29. Sci Adv. 2024. PMID: 39612338 Free PMC article.
-
The Heterotaxy Gene CCDC11 Is Important for Cytokinesis via RhoA Regulation.Cytoskeleton (Hoboken). 2025 Jun;82(6):360-371. doi: 10.1002/cm.21952. Epub 2024 Oct 31. Cytoskeleton (Hoboken). 2025. PMID: 39479942 Free PMC article.
-
Efficient proliferation and mitosis of glioblastoma cells infected with human cytomegalovirus is mediated by RhoA GTPase.Mol Med Rep. 2020 Oct;22(4):3066-3072. doi: 10.3892/mmr.2020.11434. Epub 2020 Aug 19. Mol Med Rep. 2020. PMID: 32945485 Free PMC article.
References
-
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol. 2012;199(2):365-380. https://doi.org/10.1083/jcb.201205029. - DOI - PMC - PubMed
-
- Ratheesh A, Priya R, Yap AS. Coordinating Rho and Rac. In: The Molecular Biology of Cadherins. Vol 116. Progress in Molecular Biology and Translational Science. Elsevier; 2013:49-68. https://doi.org/10.1016/B978-0-12-394311-8.00003-0. - DOI - PubMed
-
- Wennerberg K, Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. 2005;118(5):843-846. https://doi.org/10.1242/jcs.01660. - DOI - PubMed
-
- Jaffe AB, Hall A. RHO GTPASES: Biochemistry and Biology. Annu Rev Cell Dev Biol. 2005;21(1):247-269. https://doi.org/10.1146/annurev.cellbio.21.020604.150721. - DOI - PubMed
-
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: Critical Elements in the Control of Small G Proteins. Cell. 2007;129(5):865-877. https://doi.org/10.1016/j.cell.2007.05.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
