Design, Synthesis, and in Vitro and in Vivo Evaluation of Ouabain Analogues as Potent and Selective Na,K-ATPase α4 Isoform Inhibitors for Male Contraception
- PMID: 29291372
- PMCID: PMC5846083
- DOI: 10.1021/acs.jmedchem.7b00925
Design, Synthesis, and in Vitro and in Vivo Evaluation of Ouabain Analogues as Potent and Selective Na,K-ATPase α4 Isoform Inhibitors for Male Contraception
Abstract
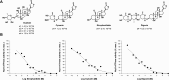
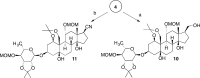
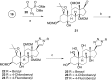
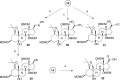
Na,K-ATPase α4 is a testis-specific plasma membrane Na+ and K+ transporter expressed in sperm flagellum. Deletion of Na,K-ATPase α4 in male mice results in complete infertility, making it an attractive target for male contraception. Na,K-ATPase α4 is characterized by a high affinity for the cardiac glycoside ouabain. With the goal of discovering selective inhibitors of the Na,K-ATPase α4 and of sperm function, ouabain derivatives were modified at the glycone (C3) and the lactone (C17) domains. Ouabagenin analogue 25, carrying a benzyltriazole moiety at C17, is a picomolar inhibitor of Na,K-ATPase α4, with an outstanding α4 isoform selectivity profile. Moreover, compound 25 decreased sperm motility in vitro and in vivo and affected sperm membrane potential, intracellular Ca2+, pH, and hypermotility. These results proved that the new ouabagenin triazole analogue is an effective and selective inhibitor of Na,K-ATPase α4 and sperm function.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



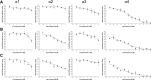



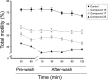


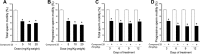
References
-
- Frank P. I.; Kay C. R.; Scott L. M.; Hannaford P. C.; Haran D. Pregnancy following induced abortion: maternal morbidity, congenital abnormalities and neonatal death. Royal College of General Practitioners/Royal College of Obstetricians and Gynaecologists Joint Study. BJOG 1987, 94, 836–842. 10.1111/j.1471-0528.1987.tb03750.x. - DOI - PubMed
-
- Michie L.; Cameron S. T. Improving the uptake of long acting reversible contraception: a review. Minerva Ginecol. 2013, 65, 241–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous

