Serotonin receptor 2B signaling with interstitial cell activation and leaflet remodeling in degenerative mitral regurgitation
- PMID: 29291394
- PMCID: PMC5856457
- DOI: 10.1016/j.yjmcc.2017.12.014
Serotonin receptor 2B signaling with interstitial cell activation and leaflet remodeling in degenerative mitral regurgitation
Abstract
Aims: Mitral valve interstitial cells (MVIC) play an important role in the pathogenesis of degenerative mitral regurgitation (MR) due to mitral valve prolapse (MVP). Numerous clinical studies have observed serotonin (5HT) dysregulation in cardiac valvulopathies; however, the impact of 5HT-mediated signaling on MVIC activation and leaflet remodeling in MVP have been investigated to a limited extent. Here we test the hypothesis that 5HT receptors (5HTRs) signaling contributes to MVP pathophysiology.
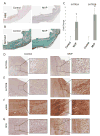
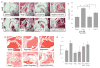
Methods and results: Diseased human MV leaflets were obtained during cardiac surgery for MVP; normal MV leaflets were obtained from heart transplants. MV RNA was used for microarray analysis of MVP patients versus control, highlighting genes that indicate the involvement of 5HTR pathways and extracellular matrix remodeling in MVP. Human MV leaflets were also studied in vitro and ex vivo with biomechanical testing to assess remodeling in the presence of a 5HTR2B antagonist (LY272015). MVP leaflets from Cavalier King Charles Spaniels were used as a naturally acquired in vivo model of MVP. These canine MVP leaflets (N=5/group) showed 5HTR2B upregulation. This study also utilized CB57.1ML/6 mice in order to determine the effect of Angiotensin II infusion on MV remodeling. Histological analysis showed that MV thickening due to chronic Angiotensin II remodeling is mitigated by a 5HTR2B antagonist (LY272015) but not by 5HTR2A inhibitors.
Conclusion: In humans, MVP is associated with an upregulation in 5HTR2B expression and increased 5HT receptor signaling in the leaflets. Antagonism of 5HTR2B mitigates MVIC activation in vitro and MV remodeling in vivo. These observations support the view that 5HTR signaling is involved not only in previously reported 5HT-related valvulopathies, but it is also involved in the pathological remodeling of MVP.
Keywords: Angiotensin; Cardiovascular disease; Mitral valve; Physiology; Serotonin; Surgery.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


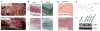

References
-
- Freed LA, Levy D, Levine RA, Evans JC, Larson MG, Fuller DL, et al. Mitral valve prolapse and atrial septal aneurysm: an evaluation in the Framingham Heart Study. Am J Cardiol. 2002;89:1326–1329. - PubMed
-
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

