Dually modified transmembrane proteoglycans in development and disease
- PMID: 29291930
- PMCID: PMC5866756
- DOI: 10.1016/j.cytogfr.2017.12.003
Dually modified transmembrane proteoglycans in development and disease
Erratum in
-
Corrigendum to "Dually modified transmembrane proteoglycans in development and disease" [Cytokine Growth Factor Rev. 39 (2018) 124-136].Cytokine Growth Factor Rev. 2022 Oct;67:105. doi: 10.1016/j.cytogfr.2022.08.002. Epub 2022 Aug 22. Cytokine Growth Factor Rev. 2022. PMID: 36008226 No abstract available.
Abstract
Aberrant cell signaling in response to secreted growth factors has been linked to the development of multiple diseases, including cancer. As such, understanding mechanisms that control growth factor availability and receptor-growth factor interaction is vital. Dually modified transmembrane proteoglycans (DMTPs), which are classified as cell surface macromolecules composed of a core protein decorated with covalently linked heparan sulfated (HS) and/or chondroitin sulfated (CS) glycosaminoglycan (GAG) chains, provide one type of regulatory mechanism. Specifically, DMTPs betaglycan and syndecan-1 (SDC1) play crucial roles in modulating key cell signaling pathways, such as Wnt, transforming growth factor-β and fibroblast growth factor signaling, to affect epithelial cell biology and cancer progression. This review outlines current and potential functions for betaglycan and SDC1, with an emphasis on comparing individual roles for HS and CS modified DMTPs. We highlight the mutual dependence of DMTPs' GAG chains and core proteins and provide comprehensive knowledge on how these DMTPs, through regulation of ligand availability and receptor internalization, control cell signaling pathways involved in development and disease.
Keywords: Betaglycan; Cancer; Cell signaling; Glycosaminoglycan; Proteoglycan; Syndecan.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
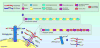
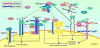


References
-
- Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

