Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form
- PMID: 29291972
- PMCID: PMC5753428
- DOI: 10.1016/j.ydbio.2017.08.032
Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form
Abstract
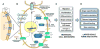
The ability to control pattern formation is critical for the both the embryonic development of complex structures as well as for the regeneration/repair of damaged or missing tissues and organs. In addition to chemical gradients and gene regulatory networks, endogenous ion flows are key regulators of cell behavior. Not only do bioelectric cues provide information needed for the initial development of structures, they also enable the robust restoration of normal pattern after injury. In order to expand our basic understanding of morphogenetic processes responsible for the repair of complex anatomy, we need to identify the roles of endogenous voltage gradients, ion flows, and electric fields. In complement to the current focus on molecular genetics, decoding the information transduced by bioelectric cues enhances our knowledge of the dynamic control of growth and pattern formation. Recent advances in science and technology place us in an exciting time to elucidate the interplay between molecular-genetic inputs and important biophysical cues that direct the creation of tissues and organs. Moving forward, these new insights enable additional approaches to direct cell behavior and may result in profound advances in augmentation of regenerative capacity.
Keywords: Bioelectricity; Ion channel; Patterning; Resting potential; Voltage.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Adams D, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007a;134:1323–1335. - PubMed
-
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007b;134:1323–1335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

