Triple Vectors Expand AAV Transfer Capacity in the Retina
- PMID: 29292161
- PMCID: PMC5835116
- DOI: 10.1016/j.ymthe.2017.11.019
Triple Vectors Expand AAV Transfer Capacity in the Retina
Abstract
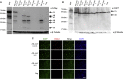
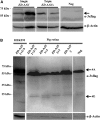
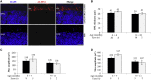
Retinal gene transfer with adeno-associated viral (AAV) vectors holds great promise for the treatment of inherited retinal degenerations (IRDs). One limit of AAV is its transfer capacity of about 5 kb, which can be expanded to about 9 kb, using dual AAV vectors. This strategy would still not suffice for treatment of IRDs such as Usher syndrome type 1D or Alström syndrome type I (ALMS) due to mutations in CDH23 or ALMS1, respectively. To overcome this limitation, we generated triple AAV vectors, with a maximal transfer capacity of about 14 kb. Transcriptomic analysis following triple AAV transduction showed the expected full-length products along a number of aberrant transcripts. However, only the full-length transcripts are efficiently translated in vivo. We additionally showed that approximately 4% of mouse photoreceptors are transduced by triple AAV vectors and showed correct localization of recombinant ALMS1. The low-photoreceptor transduction levels might justify the modest and transient improvement we observe in the retina of a mouse model of ALMS. However, the levels of transduction mediated by triple AAV vectors in pig retina reached 40% of those observed with single vectors, and this bodes well for further improving the efficiency of triple AAV vectors in the retina.
Keywords: AAV; gene therapy; large gene; retina; triple AAV.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Berger W., Kloeckener-Gruissem B., Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 2010;29:335–375. - PubMed
-
- Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

