Parental Smoking Cessation: Impacting Children's Tobacco Smoke Exposure in the Home
- PMID: 29292310
- PMCID: PMC5745674
- DOI: 10.1542/peds.2017-1026M
Parental Smoking Cessation: Impacting Children's Tobacco Smoke Exposure in the Home
Abstract
Objectives: There is no safe or risk-free level of tobacco use or tobacco smoke exposure. In this randomized controlled trial, we tested a tobacco control intervention in families and specifically evaluated a tailored cessation intervention for the parents and/or caregivers (Ps/Cs) who were smokers while their children were simultaneously enrolled in tobacco prevention.
Methods: Ps/Cs and children were recruited from 14 elementary schools across rural and urban settings. Approximately one-fourth (24.3%; n = 110) of the total Ps/Cs enrolled in the randomized controlled trial (n = 453) were smokers, predominantly women (80.9%), with a mean age of 37.7 years. (SD 12.2); 62.7% were African American, 44% had less than a high school education, and 58% earned <$20 000 annually. P/C smokers were offered a tailored cessation intervention in years 1 and 2. Self-report smoking status and saliva cotinine were obtained at baseline, the end of treatment (EOT) and/or year 2, and in the year 4 follow-up.
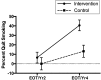
Results: Ps/Cs in the intervention group showed a larger increase in self-reported smoking abstinence over time (EOT: 6.5% [SE = 5.7%]; year 4: 40.6% [SE = 5.7%]) than the control group (EOT: 0.0% [SE = 6.5%]; year 4: 13.2% [SE = 6.4%]; F = 4.82; P = .0306). For cotinine, the intervention group showed a decrease from baseline (239.9 [SE = 1.3]) to EOT 99.3 [SE = 1.4]) and then maintenance through year 4 (109.6 [SE = 1.4]), whereas the control group showed increases from baseline (221.1 [SE = 1.4]) to EOT (239.0 [SE = 1.4]) to year 4 (325.8 [SE = 14]; F = 5.72; P = .0039).
Conclusions: This study provides evidence that tailored cessation offered to Ps/Cs in their children's schools during their children's enrollment in tobacco prevention may contribute to more robust success in P/C cessation and a reduction of tobacco smoke exposure in children.
Trial registration: ClinicalTrials.gov NCT01164306.
Copyright © 2018 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures
References
-
- US Department of Health and Human Services The Health Consequences of Smoking—50 years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014
-
- Institute of Medicine Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. Washington, DC: National Academy of Sciences, Institute of Medicine; 2009
-
- National Toxicology Program Report on Carcinogens. 13th ed. Washington, DC: US Department of Health and Human Services, Public Health Service; 2014
-
- US Department of Health and Human Services A Report of the Surgeon General: How Tobacco Smoke Causes Disease: What It Means to You. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010
-
- US Department of Health and Human Services The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical