Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces
- PMID: 29292334
- PMCID: PMC6424438
- DOI: 10.1302/0301-620X.100B1.BJJ-2017-0551.R1
Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces
Abstract
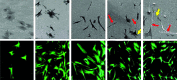
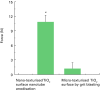
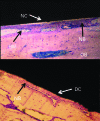
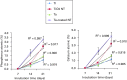
The development and pre-clinical evaluation of nano-texturised, biomimetic, surfaces of titanium (Ti) implants treated with titanium dioxide (TiO2) nanotube arrays is reviewed. In vitro and in vivo evaluations show that TiO2 nanotubes on Ti surfaces positively affect the osseointegration, cell differentiation, mineralisation, and anti-microbial properties. This surface treatment can be superimposed onto existing macro and micro porous Ti implants creating a surface texture that also interacts with cells at the nano level. Histology and mechanical pull-out testing of specimens in rabbits indicate that TiO2 nanotubes improves bone bonding nine-fold (p = 0.008). The rate of mineralisation associated with TiO2 nanotube surfaces is about three times that of non-treated Ti surfaces. In addition to improved osseointegration properties, TiO2 nanotubes reduce the initial adhesion and colonisation of Staphylococcus epidermidis Collectively, the properties of Ti implant surfaces enhanced with TiO2 nanotubes show great promise. Cite this article: Bone Joint J 2018;100-B(1 Supple A):9-16.
Keywords: Antimicrobial; Mineralisation; Nano-texturing; Nanotubes; Osseointegration; Titanium dioxide.
©2018 The British Editorial Society of Bone & Joint Surgery.
Figures




References
-
- Long M, Rack HJ. Titanium alloys in total joint replacement – a materials science perspective. Biomaterials 1998;19:1621–1639. - PubMed
-
- Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010;31:706–713. - PubMed
-
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012;379:1331–1340. - PubMed
-
- Dalury DF. Cementless total knee arthroplasty. Bone Joint J 2016; 98-B:867–873. - PubMed
-
- Drees P, Eckardt A, Gay RE, Gay S, Huber LC. Mechanisms of disease: molecular insights into aseptic loosening of orthopedic implants. Nat Clin Pract Rheumatol 2007;3:165–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

