Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus
- PMID: 29292384
- PMCID: PMC5972547
- DOI: 10.1038/s41564-017-0081-7
Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus
Abstract
Epstein-Barr virus (EBV) is an oncogenic virus that infects more than 90% of the world's population 1 . EBV predominantly infects human B cells and epithelial cells, which is initiated by fusion of the viral envelope with a host cellular membrane 2 . The mechanism of EBV entry into B cells has been well characterized 3 . However, the mechanism for epithelial cell entry remains elusive. Here, we show that the integrins αvβ5, αvβ6 and αvβ8 do not function as entry and fusion receptors for epithelial cells, whereas Ephrin receptor tyrosine kinase A2 (EphA2) functions well for both. EphA2 overexpression significantly increased EBV infection of HEK293 cells. Using a virus-free cell-cell fusion assay, we found that EphA2 dramatically promoted EBV but not herpes simplex virus (HSV) fusion with HEK293 cells. EphA2 silencing using small hairpin RNA (shRNA) or knockout by CRISPR-Cas9 blocked fusion with epithelial cells. This inhibitory effect was rescued by the expression of EphA2. Antibody against EphA2 blocked epithelial cell infection. Using label-free surface plasmon resonance binding studies, we confirmed that EphA2 but not EphA4 specifically bound to EBV gHgL and this interaction is through the EphA2 extracellular domain (ECD). The discovery of EphA2 as an EBV epithelial cell receptor has important implications for EBV pathogenesis and may uncover new potential targets that can be used for the development of novel intervention strategies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
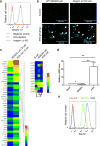
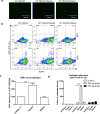
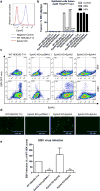

References
-
- Longnecker R, Kieff E, Cohen JI. Fields Viorology. 6. Lippincott Williams & Wilkins; Philadelphia, PA: 2013.
-
- Epstein MA, Achong BG, Barr YM. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;1(7335):702–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

