Mesenchymal Stem Cell-Conditioned Medium Modulates Apoptotic and Stress-Related Gene Expression, Ameliorates Maturation and Allows for the Development of Immature Human Oocytes after Artificial Activation
- PMID: 29292728
- PMCID: PMC5748689
- DOI: 10.3390/genes8120371
Mesenchymal Stem Cell-Conditioned Medium Modulates Apoptotic and Stress-Related Gene Expression, Ameliorates Maturation and Allows for the Development of Immature Human Oocytes after Artificial Activation
Abstract
The aim of the present study was to determine whether mesenchymal stem cell-conditioned medium (MSC-CM) modulates apoptotic and stress-related gene expression, and ameliorates maturation and developmental potential of immature human oocytes after artificial activation. A total of 247 surplus immature germinal vesicle (GV) oocytes obtained from infertile women were allocated into two in vitro maturation (IVM) groups: 1: GV oocytes (n = 116) matured in vitro (fIVM), and 2: GV oocytes (n = 131) that were vitrified, then in vitro matured (vIVM). Also, two maturation media were used: Alpha-minimum essential medium (α-MEM) and human umbilical cord-derived MSCs (hUCM). After 36 h of incubation, the IVM oocytes were examined for nuclear maturation. In IVM-matured oocytes, cytoplasmic maturation was evaluated after artificial activation through Ionomycin. Moreover, the quantitative expressions of B-cell CLL/lymphoma 2 (BCL2), BCL2-associated X protein (BAX), superoxide dismutase (SOD), and Heat shock proteins (HSP70) in matured oocytes were assessed by quantitative Real-time polymerase chain reaction (qRT-PCR) and compared with fresh and vitrified in vivo matured oocytes, which were used as fIVM and vIVM controls, respectively. The highest maturation rate was found in hUCM in fIVM, and the lowest maturation rate was found using α-MEM in vIVM (85.18% and 71.42%, respectively). The cleavage rate in fIVM was higher than that in vIVM (83.4% vs. 72.0%). In addition, the cleavage rate in α-MEM was lower than that in the hUCM (66.0% vs. 89.4%). Furthermore, the difference between parthenote embryo arrested in 4-8 cells (p < 0.04) and the quality of embryo arrested in 8-cell (p < 0.007) were significant. The developmental stages of parthenote embryos in hUCM versus α-MEM were as follows: 2-4 cell (89.45% vs. 66.00%, respectively), 4-8 cell (44.31% vs. 29.11%, respectively), morula (12.27% vs. 2.63%, respectively), and blastocysts (2.5% vs. 0%, respectively). The messenger RNA (mRNA) expression levels of BCL2, BAX and SOD were significantly different (p < 0.05) between the matured IVM oocytes. Overall, hUCM showed potential efficacy in terms of ameliorating oocyte maturation and in promoting the development and mRNA expression of BAX, BCL2, and SOD.
Keywords: conditioned medium; gene expression; in vitro oocyte; mesenchymal stem cells; oocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
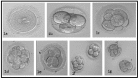
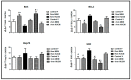
References
-
- Ferreira E.M., Vireque A.A., Adona P.R., Ferriani R.A., Navarro P.A. Prematuration of bovine oocytes with butyrolactone I reversibly arrests meiosis without increasing meiotic abnormalities after in vitro maturation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;145:76–80. doi: 10.1016/j.ejogrb.2009.03.016. - DOI - PubMed
-
- Somfai T., Ozawa M., Noguchi J., Kaneko H., Karja N.W.K., Farhudin M., Dinnyés A., Nagai T., Kikuchi K. Developmental competence of in vitro-fertilized porcine oocytes after in vitro maturation and solid surface vitrification: Effect of cryopreservation on oocyte antioxidative system and cell cycle stage. Cryobiology. 2007;55:115–126. doi: 10.1016/j.cryobiol.2007.06.008. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

