Endoplasmic reticulum stress in the pathogenesis of fibrotic disease
- PMID: 29293089
- PMCID: PMC5749533
- DOI: 10.1172/JCI93560
Endoplasmic reticulum stress in the pathogenesis of fibrotic disease
Abstract
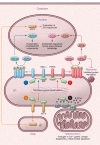
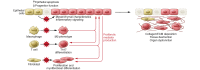
Eukaryotic cells contain an elegant protein quality control system that is crucial in maintaining cellular homeostasis; however, dysfunction of this system results in endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR). Severe or prolonged ER stress is associated with the development of degenerative and fibrotic disorders in multiple organs, as evidenced by the identification of disease-causing mutations in epithelial-restricted genes that lead to protein misfolding or mistrafficking in familial fibrotic diseases. Emerging evidence implicates ER stress and UPR signaling in a variety of profibrotic mechanisms in individual cell types. In epithelial cells, ER stress can induce apoptosis, inflammatory signaling, and epithelial-mesenchymal transition. In other cell types, ER stress is linked to myofibroblast activation, macrophage polarization, and T cell differentiation. ER stress-targeted therapies have begun to emerge using approaches that range from global enhancement of chaperone function to selective targeting of activated ER stress sensors and other downstream mediators. As the complex regulatory mechanisms of this system are further clarified, there are opportunities to develop new disease-modifying therapeutic strategies in a wide range of chronic fibrotic diseases.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

