Challenges and new frontiers in analytical characterization of antibody-drug conjugates
- PMID: 29293399
- PMCID: PMC5825200
- DOI: 10.1080/19420862.2017.1412025
Challenges and new frontiers in analytical characterization of antibody-drug conjugates
Abstract
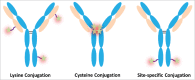
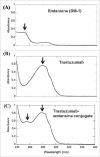
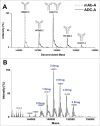
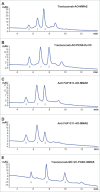
Antibody-drug conjugates (ADCs) are a growing class of biotherapeutics in which a potent small molecule is linked to an antibody. ADCs are highly complex and structurally heterogeneous, typically containing numerous product-related species. One of the most impactful steps in ADC development is the identification of critical quality attributes to determine product characteristics that may affect safety and efficacy. However, due to the additional complexity of ADCs relative to the parent antibodies, establishing a solid understanding of the major quality attributes and determining their criticality are a major undertaking in ADC development. Here, we review the development challenges, especially for reliable detection of quality attributes, citing literature and new data from our laboratories, highlight recent improvements in major analytical techniques for ADC characterization and control, and discuss newer techniques, such as two-dimensional liquid chromatography, that have potential to be included in analytical control strategies.
Keywords: Antibody-drug conjugates; CQA, DAR; analytical; characterization; quality attribute; stability.
Figures

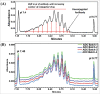
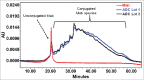
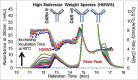
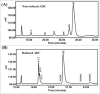
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
