Left atrial voltage, circulating biomarkers of fibrosis, and atrial fibrillation ablation. A prospective cohort study
- PMID: 29293545
- PMCID: PMC5749720
- DOI: 10.1371/journal.pone.0189936
Left atrial voltage, circulating biomarkers of fibrosis, and atrial fibrillation ablation. A prospective cohort study
Abstract
Aims: To test the ability of four circulating biomarkers of fibrosis, and of low left atrial voltage, to predict recurrence of atrial fibrillation after catheter ablation.
Background: Circulating biomarkers potentially may be used to improve patient selection for atrial fibrillation ablation. Low voltage areas in the left atrium predict arrhythmia recurrence when mapped in sinus rhythm. This study tested type III procollagen N terminal peptide (PIIINP), galectin-3 (gal-3), fibroblast growth factor 23 (FGF-23), and type I collagen C terminal telopeptide (ICTP), and whether low voltage areas in the left atrium predicted atrial fibrillation recurrence, irrespective of the rhythm during mapping.
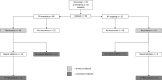
Methods: 92 atrial fibrillation ablation patients were studied. Biomarker levels in peripheral and intra-cardiac blood were measured with enzyme-linked immunosorbent assay. Low voltage (<0.5mV) was expressed as a proportion of the mapped left atrial surface area. Follow-up was one year. The primary endpoint was recurrence of arrhythmia. The secondary endpoint was a composite of recurrence despite two procedures, or after one procedure if no second procedure was undertaken.
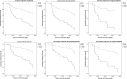
Results: The biomarkers were not predictive of either endpoint. After multivariate Cox regression analysis, high proportion of low voltage area in the left atrium was found to predict the primary endpoint in sinus rhythm mapping (hazard ratio 4.323, 95% confidence interval 1.337-13.982, p = 0.014) and atrial fibrillation mapping (hazard ratio 5.195, 95% confidence interval 1.032-26.141, p = 0.046). This effect was also apparent for the secondary endpoint.
Conclusion: The studied biomarkers do not predict arrhythmia recurrence after catheter ablation. Left atrial voltage is an independent predictor of recurrence, whether the left atrium is mapped in atrial fibrillation or sinus rhythm.
Conflict of interest statement
Figures

References
-
- Parkash R, Tang AS, Sapp JL, Wells G. Approach to the catheter ablation technique of paroxysmal and persistent atrial fibrillation: a meta-analysis of the randomized controlled trials. Journal of cardiovascular electrophysiology. 2011;22(7):729–38. doi: 10.1111/j.1540-8167.2011.02010.x . - DOI - PubMed
-
- Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. The New England journal of medicine. 2015;372(19):1812–22. doi: 10.1056/NEJMoa1408288 . - DOI - PubMed
-
- Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. Journal of the American College of Cardiology. 2015;66(8):943–59. doi: 10.1016/j.jacc.2015.06.1313 . - DOI - PubMed
-
- Begg GA, Holden AV, Lip GY, Plein S, Tayebjee MH. Assessment of atrial fibrosis for the rhythm control of atrial fibrillation. International journal of cardiology. 2016;220:155–61. doi: 10.1016/j.ijcard.2016.06.144 . - DOI - PubMed
-
- Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. Journal of the American College of Cardiology. 2005;45(2):285–92. doi: 10.1016/j.jacc.2004.10.035 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

