Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple-negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313)
- PMID: 29293876
- PMCID: PMC5888953
- DOI: 10.1093/annonc/mdx821
Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple-negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313)
Abstract
Background: Homologous recombination deficiency (HRD)-causing alterations have been reported in triple-negative breast cancer (TNBC). We hypothesized that TNBCs with HRD alterations might be more sensitive to anthracycline plus cyclophosphamide-based chemotherapy and report on HRD status and BRCA1 promoter methylation (PM) as prognostic markers in TNBC patients treated with adjuvant doxorubicin (A) and cyclophosphamide (C) in SWOG9313.
Patients and methods: In total, 425 TNBC patients were identified from S9313. HRD score, tumor BRCA1/2 sequencing, and BRCA1 PM were carried out on DNA isolated from formalin-fixed paraffin-embedded tissue. Positive HRD status was defined as either a deleterious tumor BRCA1/2 (tBRCA) mutation or a pre-defined HRD score ≥42. Markers were tested for prognostic value on disease-free survival (DFS) and overall survival (OS) using Cox regression models adjusted for treatment assignment and nodal status.
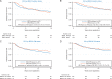
Results: HRD status was determined in 89% (379/425) of cases. Of these, 67% were HRD positive (27% with tBRCA mutation, 40% tBRCA-negative but HRD score ≥42). HRD-positive status was associated with a better DFS [hazard ratio (HR) 0.72; 95% confidence interval (CI) 0.51-1.00; P = 0.049] and non-significant trend toward better OS (HR = 0.71; 95% CI 0.48-1.03; P = 0.073). High HRD score (≥42) in tBRCA-negative patients (n = 274) was also associated with better DFS (HR = 0.64; 95% CI 0.43-0.94; P = 0.023) and OS (HR = 0.65; 95% CI 0.42-1.00; P = 0.049). BRCA1 PM was evaluated successfully in 82% (348/425) and detected in 32% of cases. The DFS HR for BRCA1 PM was similar to that for HRD but did not reach statistical significance (HR = 0.79; 95% CI 0.54-1.17; P = 0.25).
Conclusions: HRD positivity was observed in two-thirds of TNBC patients receiving adjuvant AC and was associated with better DFS. HRD status may identify TNBC patients who receive greater benefit from AC-based chemotherapy and should be evaluated further in prospective studies.
Clinical trials number: Int0137 (The trial pre-dates Clinicaltrial.Gov website establishment).
Figures

References
-
- Liedtke C, Mazouni C, Hess KR. et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26(8): 1275–1281. - PubMed
-
- Haffty BG, Yang Q, Reiss M. et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. JCO 2006; 24: 5652–5657. - PubMed
-
- Tan DS, Marchio C, Jones RL. et al. Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat 2008; 111(1): 27–44. - PubMed
-
- Isakoff SJ, Goss PE, Mayer EL. et al. Impact of BRCA1/2 mutation status in TBCRC009: A multicenter phase II study of cisplatin or carboplatin for metastatic triple negative breast cancer. Cancer Res 2012; 72(24 Suppl): 140s–141s.
-
- Tutt A, Robson M, Garber JE. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010; 376(9737): 235–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

