The Herb-Drug Pharmacokinetic Interaction of 5-Fluorouracil and Its Metabolite 5-Fluoro-5,6-Dihydrouracil with a Traditional Chinese Medicine in Rats
- PMID: 29295501
- PMCID: PMC5795976
- DOI: 10.3390/ijms19010025
The Herb-Drug Pharmacokinetic Interaction of 5-Fluorouracil and Its Metabolite 5-Fluoro-5,6-Dihydrouracil with a Traditional Chinese Medicine in Rats
Abstract
Background: Xiang-Sha-Liu-Jun-Zi-Tang (XSLJZT) is the most common traditional formula given to colorectal and breast cancer patients in Taiwan, according to a statistical study of the National Health Insurance Research Database. 5-Fluorouracil (5-FU) is widely used as the first line of treatment for colorectal cancer. Thus, the aim of study is to investigate the pharmacokinetic interaction of XSLJZT and 5-FU.
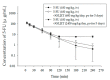
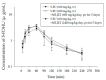
Methods: To investigate the herb-drug interaction of XSLJZT with 5-FU as well as its metabolite 5-fluoro-5,6-dihydrouracil (5-FDHU) using pharmacokinetics, a high-performance liquid chromatography (HPLC) system coupled with a photodiode array detector was developed to monitor 5-FU and 5-FDHU levels in rat blood. Rats were divided into three cohorts, one of which was administered 5-FU (100 mg/kg, iv-intravenous) alone, while the other two groups were pretreated with low and high doses of XSLJZT (600 mg/kg/day or 2400 mg/kg/day for 5 consecutive days) in combination with 5-FU.
Results: The results demonstrated that 5-FU level was not significantly different between the group treated with only 5-FU and the group pretreated with a normal dose of XSLJZT (600 mg/kg/day). However, pharmacokinetic analysis revealed that pretreatment with a high dose of XSLJZT (2400 mg/kg/day) extended the residence time and increased the volume of distribution of 5-FU. No significant distinctions were found in 5-FDHU pharmacokinetic parameters at three doses of XSLJZT.
Conclusions: Overall, the pharmacokinetic results confirm the safety of coadministering 5-FU with XSLJZT, and provide practical dosage information for clinical practice.
Keywords: 5-fluoro-5,6-dihydrouracil (5-FDHU); 5-fluorouracil (5-FU); HPLC–UV; Xiang-Sha-Liu-Jun-Zi-Tang (XSLJZT); herb–drug interaction; pharmacokinetic; traditional Chinese medicine (TCM).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Büchel B., Rhyn P., Schürch S., Bühr C., Amstutz U., Largiadèr C.R. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed. Chromatogr. 2013;27:7–16. doi: 10.1002/bmc.2741. - DOI - PubMed
-
- Yamada T., Tanaka N., Yokoi K., Ishikawa N., Seya T., Horiba K., Kanazawa Y., Shirakawa T., Ohkawa K., Kudoh H., et al. Prediction of sensitivity to 5-fluorouracil (5-fu) by metabolic and target enzyme activities in colon cancer. Gan to Kagaku Ryoho. 2006;33:1603–1609. - PubMed
-
- Levy E., Piedbois P., Buyse M., Pignon J., Rougier P., Ryan L., Hansen R., Zee B., Weinerman B., Pater J. Toxicity of fluorouracil in patients with advanced colorectal cancer: Effect of administration schedule and prognostic factors. J. Clin. Oncol. 1998;16:3537–3541. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

