Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3'-Dichlorobiphenyl (PCB 11)
- PMID: 29295518
- PMCID: PMC5874777
- DOI: 10.3390/toxics6010004
Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3'-Dichlorobiphenyl (PCB 11)
Abstract
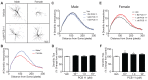
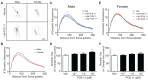
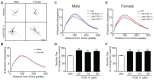
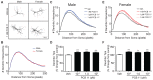
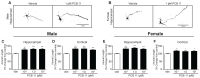
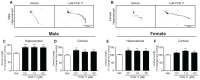
PCB 11 is an emerging global pollutant that we recently showed promotes axonal and dendritic growth in primary rat neuronal cell cultures. Here, we address the influence of sex and species on neuronal responses to PCB 11. Neuronal morphology was quantified in sex-specific primary hippocampal and cortical neuron-glia co-cultures derived from neonatal C57BL/6J mice and Sprague Dawley rats exposed for 48 h to vehicle (0.1% DMSO) or PCB 11 at concentrations ranging from 1 fM to 1 nM. Total axonal length was quantified in tau-1 immunoreactive neurons at day in vitro (DIV) 2; dendritic arborization was assessed by Sholl analysis at DIV 9 in neurons transfected with MAP2B-FusRed. In mouse cultures, PCB 11 enhanced dendritic arborization in female, but not male, hippocampal neurons and male, but not female, cortical neurons. In rat cultures, PCB 11 promoted dendritic arborization in male and female hippocampal and cortical neurons. PCB 11 also increased axonal growth in mouse and rat neurons of both sexes and neuronal cell types. These data demonstrate that PCB 11 exerts sex-specific effects on neuronal morphogenesis that vary depending on species, neurite type, and neuronal cell type. These findings have significant implications for risk assessment of this emerging developmental neurotoxicant.
Keywords: PCB 11; axons; dendrites; developmental neurotoxicity; in vitro; neuronal morphogenesis; sex bias.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors of this work had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Sex-Dependent Effects of 2,2',3,5',6-Pentachlorobiphenyl on Dendritic Arborization of Primary Mouse Neurons.Toxicol Sci. 2019 Mar 1;168(1):95-109. doi: 10.1093/toxsci/kfy277. Toxicol Sci. 2019. PMID: 30395321 Free PMC article.
-
3,3'-Dichlorobiphenyl (PCB 11) promotes dendritic arborization in primary rat cortical neurons via a CREB-dependent mechanism.Arch Toxicol. 2018 Nov;92(11):3337-3345. doi: 10.1007/s00204-018-2307-8. Epub 2018 Sep 17. Arch Toxicol. 2018. PMID: 30225637 Free PMC article.
-
Detection of 3,3'-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons.Toxicol Sci. 2017 Aug 1;158(2):401-411. doi: 10.1093/toxsci/kfx100. Toxicol Sci. 2017. PMID: 28510766 Free PMC article.
-
PCB 95 promotes dendritic growth in primary rat hippocampal neurons via mTOR-dependent mechanisms.Arch Toxicol. 2018 Oct;92(10):3163-3173. doi: 10.1007/s00204-018-2285-x. Epub 2018 Aug 21. Arch Toxicol. 2018. PMID: 30132043 Free PMC article.
-
Methods for shipping live primary cortical and hippocampal neuron cultures from postnatal mice.Curr Res Neurobiol. 2022 Dec 17;4:100069. doi: 10.1016/j.crneur.2022.100069. eCollection 2023. Curr Res Neurobiol. 2022. PMID: 36589676 Free PMC article. Review.
Cited by
-
The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): similarities and differences.Environ Sci Pollut Res Int. 2020 Mar;27(9):8885-8896. doi: 10.1007/s11356-019-06723-5. Epub 2019 Nov 12. Environ Sci Pollut Res Int. 2020. PMID: 31713823 Free PMC article. Review.
-
Developmental polychlorinated biphenyl (PCB) exposure alters voiding physiology in young adult male and female mice.Am J Clin Exp Urol. 2022 Apr 15;10(2):82-97. eCollection 2022. Am J Clin Exp Urol. 2022. PMID: 35528463 Free PMC article.
-
Sex-Dependent Effects of 2,2',3,5',6-Pentachlorobiphenyl on Dendritic Arborization of Primary Mouse Neurons.Toxicol Sci. 2019 Mar 1;168(1):95-109. doi: 10.1093/toxsci/kfy277. Toxicol Sci. 2019. PMID: 30395321 Free PMC article.
-
Sex-specific effects of developmental exposure to polychlorinated biphenyls on neuroimmune and dopaminergic endpoints in adolescent rats.Neurotoxicol Teratol. 2020 May-Jun;79:106880. doi: 10.1016/j.ntt.2020.106880. Epub 2020 Apr 4. Neurotoxicol Teratol. 2020. PMID: 32259577 Free PMC article.
-
Placenta and fetal brain share a neurodevelopmental disorder DNA methylation profile in a mouse model of prenatal PCB exposure.Cell Rep. 2022 Mar 1;38(9):110442. doi: 10.1016/j.celrep.2022.110442. Cell Rep. 2022. PMID: 35235788 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

