Characterizing Metastatic HER2-Positive Gastric Cancer at the CDH1 Haplotype
- PMID: 29295527
- PMCID: PMC5795997
- DOI: 10.3390/ijms19010047
Characterizing Metastatic HER2-Positive Gastric Cancer at the CDH1 Haplotype
Abstract
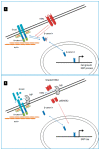
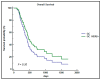
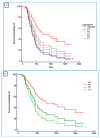
The CDH1 gene, coding for the E-cadherin protein, is linked to gastric cancer (GC) susceptibility and tumor invasion. The human epidermal growth factor receptor 2 (HER2) is amplified and overexpressed in a portion of GC. HER2 is an established therapeutic target in metastatic GC (mGC). Trastuzumab, in combination with various chemotherapeutic agents, is a standard treatment for these tumors leading to outcome improvement. Unfortunately, the survival benefit is limited to a fraction of patients. The aim of this study was to improve knowledge of the HER2 and the E-cadherin alterations in the context of GC to characterize subtypes of patients that could better benefit from targeted therapy. An association between the P7-CDH1 haplotype, including two polymorphisms (rs16260A-rs1801552T) and a subset of HER2-positive mGC with better prognosis was observed. Results indicated the potential evaluation of CDH1 haplotypes in mGC to stratify patients that will benefit from trastuzumab-based treatments. Moreover, data may have implications to understanding the HER2 and the E-cadherin interactions in vivo and in response to treatments.
Keywords: CDH1; E-cadherin; HER2; metastatic gastric cancer; rs16260; rs1801552.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Santeufemia D.A., Lumachi F., Fadda G.M., Lo Re G., Miolo G., Basso S.M.M., Chiara G.B., Tumolo S. Comment on “Repetitive transarterial chemoembolization (TACE) of liver metastases from gastric cancer: Local control and survival results”: Will there be clinical implications in the future? Eur. J. Radiol. 2013;82:1591–1592. doi: 10.1016/j.ejrad.2013.04.002. - DOI - PubMed
-
- Catalano V., Graziano F., Santini D., D’Emidio S., Baldelli A.M., Rossi D., Vincenzi B., Giordani P., Alessandroni P., Testa E., et al. Second-line chemotherapy for patients with advanced gastric cancer: Who may benefit? Br. J. Cancer. 2008;99:1402–1407. doi: 10.1038/sj.bjc.6604732. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

