Identification of Immunoreactive Peptides of Toxins to Simultaneously Assess the Neutralization Potency of Antivenoms against Neurotoxicity and Cytotoxicity of Naja atra Venom
- PMID: 29295601
- PMCID: PMC5793097
- DOI: 10.3390/toxins10010010
Identification of Immunoreactive Peptides of Toxins to Simultaneously Assess the Neutralization Potency of Antivenoms against Neurotoxicity and Cytotoxicity of Naja atra Venom
Abstract
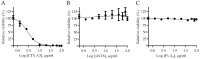
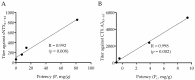
Assessing the neutralization capability of nonlethal but medically relevant toxins in venom has been a challenging task. Nowadays, neutralization efficacy is evaluated based simply on the survival rates of animals injected with antivenom together with a predefined dose of venom, which can determine potency against neurotoxicity but not validate the capability to neutralize cytotoxin-induced complications. In this study, a high correlation with in-vivo and in-vitro neutralization assays was established using the immunoreactive peptides identified from short-chain neurotoxin and cytotoxin A3. These peptides contain conserved residues associated with toxin activities and a competition assay indicated that these peptides could specifically block the antibody binding to toxin and affect the neutralization potency of antivenom. Moreover, the titers of peptide-specific antibody in antivenoms or mouse antisera were determined by enzyme-linked immunosorbent assay (ELISA) simultaneously, and the results indicated that Taiwanese bivalent antivenom (BAV) and Vietnamese snake antivenom-Naja (SAV-Naja) exhibited superior neutralization potency against the lethal effect of short-chain neurotoxin (sNTX) and cytotoxicity of cardiotoxin/cytotoxin (CTX), respectively. Thus, the reported peptide ELISA shows not only its potential for antivenom prequalification use, but also its capability of justifying the cross-neutralization potency of antivenoms against Naja atra venom toxicity.
Keywords: ELISA; Naja atra; antivenom; assessment; immunoreactive peptide; neutralization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- WHO Offset Publication . Progress in the Characterization of Venoms and Standardization of Antivenoms. Volume 58. WHO Offset Publication; Geneva, Switzerland: 1981. pp. 1–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

