Integration of Transplanted Neural Precursors with the Injured Cervical Spinal Cord
- PMID: 29295654
- PMCID: PMC6033309
- DOI: 10.1089/neu.2017.5451
Integration of Transplanted Neural Precursors with the Injured Cervical Spinal Cord
Abstract
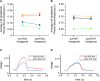
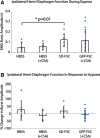
Cervical spinal cord injuries (SCI) result in devastating functional consequences, including respiratory dysfunction. This is largely attributed to the disruption of phrenic pathways, which control the diaphragm. Recent work has identified spinal interneurons as possible contributors to respiratory neuroplasticity. The present work investigated whether transplantation of developing spinal cord tissue, inherently rich in interneuronal progenitors, could provide a population of new neurons and growth-permissive substrate to facilitate plasticity and formation of novel relay circuits to restore input to the partially denervated phrenic motor circuit. One week after a lateralized, C3/4 contusion injury, adult Sprague-Dawley rats received allografts of dissociated, developing spinal cord tissue (from rats at gestational days 13-14). Neuroanatomical tracing and terminal electrophysiology was performed on the graft recipients 1 month later. Experiments using pseudorabies virus (a retrograde, transynaptic tracer) revealed connections from donor neurons onto host phrenic circuitry and from host, cervical interneurons onto donor neurons. Anatomical characterization of donor neurons revealed phenotypic heterogeneity, though donor-host connectivity appeared selective. Despite the consistent presence of cholinergic interneurons within donor tissue, transneuronal tracing revealed minimal connectivity with host phrenic circuitry. Phrenic nerve recordings revealed changes in burst amplitude after application of a glutamatergic, but not serotonergic antagonist to the transplant, suggesting a degree of functional connectivity between donor neurons and host phrenic circuitry that is regulated by glutamatergic input. Importantly, however, anatomical and functional results were variable across animals, and future studies will explore ways to refine donor cell populations and entrain consistent connectivity.
Keywords: interneurons; plasticity; respiration; spinal cord injury; transplantation.
Conflict of interest statement
No competing financial interests exist.
Figures

Similar articles
-
Anatomical Recruitment of Spinal V2a Interneurons into Phrenic Motor Circuitry after High Cervical Spinal Cord Injury.J Neurotrauma. 2017 Nov 1;34(21):3058-3065. doi: 10.1089/neu.2017.5045. Epub 2017 Jun 29. J Neurotrauma. 2017. PMID: 28548606 Free PMC article.
-
Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury.J Neurotrauma. 2018 Dec 15;35(24):2883-2903. doi: 10.1089/neu.2017.5439. Epub 2018 Aug 10. J Neurotrauma. 2018. PMID: 29873284 Free PMC article.
-
Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat.J Comp Neurol. 2008 Dec 10;511(5):692-709. doi: 10.1002/cne.21864. J Comp Neurol. 2008. PMID: 18924146 Free PMC article.
-
Spinal circuitry and respiratory recovery following spinal cord injury.Respir Physiol Neurobiol. 2009 Nov 30;169(2):123-32. doi: 10.1016/j.resp.2009.08.007. Epub 2009 Aug 19. Respir Physiol Neurobiol. 2009. PMID: 19698805 Free PMC article. Review.
-
Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons.Respir Physiol Neurobiol. 2016 Jun;226:128-36. doi: 10.1016/j.resp.2015.10.009. Epub 2015 Oct 23. Respir Physiol Neurobiol. 2016. PMID: 26506253 Free PMC article. Review.
Cited by
-
Challenges and Efficacy of Astrocyte-to-Neuron Reprogramming in Spinal Cord Injury: In Vitro Insights and In Vivo Outcomes.bioRxiv [Preprint]. 2025 Jan 21:2024.03.25.586619. doi: 10.1101/2024.03.25.586619. bioRxiv. 2025. PMID: 38585866 Free PMC article. Preprint.
-
Transneuronal tracing to map connectivity in injured and transplanted spinal networks.Exp Neurol. 2022 May;351:113990. doi: 10.1016/j.expneurol.2022.113990. Epub 2022 Jan 25. Exp Neurol. 2022. PMID: 35085573 Free PMC article. Review.
-
Incorporating Combinatorial Approaches to Encourage Targeted Neural Stem/Progenitor Cell Integration Following Transplantation in Spinal Cord Injury.Stem Cells Transl Med. 2023 Apr 17;12(4):207-214. doi: 10.1093/stcltm/szad008. Stem Cells Transl Med. 2023. PMID: 36892546 Free PMC article. Review.
-
Cell transplantation to repair the injured spinal cord.Int Rev Neurobiol. 2022;166:79-158. doi: 10.1016/bs.irn.2022.09.008. Epub 2022 Nov 9. Int Rev Neurobiol. 2022. PMID: 36424097 Free PMC article. No abstract available.
-
Combinatorial strategies for cell transplantation in traumatic spinal cord injury.Front Neurosci. 2024 Mar 6;18:1349446. doi: 10.3389/fnins.2024.1349446. eCollection 2024. Front Neurosci. 2024. PMID: 38510468 Free PMC article. Review.
References
-
- Bötel U., Gläser E., Niedeggen A., and Meindl R. (1997). The cost of ventilator-dependent spinal cord injuries-patients in the hospital and at home. Spinal Cord 35, 40–42 - PubMed
-
- National Spinal Cord Injury Statistical Center. (2017). Spinal Cord Injury Facts and Figures at a Glance. University of Alabama at Birmingham: Birmingham, AL
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

