A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea
- PMID: 29295825
- PMCID: PMC5867402
- DOI: 10.1136/annrheumdis-2017-211631
A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea
Abstract
Background: Intravenous belimumab plus standard of care (SoC) is approved in the USA and Europe for treatment of active, autoantibody-positive systemic lupus erythematosus (SLE).
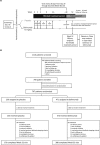
Methods: This phase III, multicentre, randomised, double-blind, placebo-controlled study (BEL113750; NCT01345253) was conducted in 49 centres across China, Japan and South Korea (May 2011-September 2015). Patients with SLE were randomised 2:1 to intravenous belimumab 10 mg/kg or placebo, plus SoC, every 4 weeks until Week 48. The primary endpoint was the SLE Responder Index (SRI) 4 response rate at Week 52. Secondary endpoints were the percentage of patients with ≥4 point reduction in Safety of Oestrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI), SRI7, time to first severe flare and number of days prednisone (or equivalent) dose ≤7.5 mg/day and/or reduced by 50% from baseline. Safety was assessed.
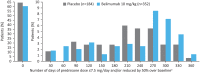
Results: The modified intent-to-treat population included 677 patients (belimumab n=451, placebo n=226). At Week 52, the SRI4 response rate was higher with belimumab versus placebo (53.8% vs 40.1%; OR: 1.99 (95% CI: 1.40, 2.82; P=0.0001)). The percentages of patients with a ≥4 point reduction in SELENA-SLEDAI and an SRI7 response were significantly greater for belimumab versus placebo. Patients in the belimumab group had a 50% lower risk of experiencing a severe flare than those receiving placebo (P=0.0004). In patients with baseline prednisone dose >7.5 mg/day, there was a significant reduction in steroid use favouring belimumab (P=0.0228). The incidence of adverse events was similar between groups.
Conclusions: In patients with SLE from North East Asia, belimumab significantly improved disease activity, while reducing prednisone use, with no new safety issues.
Keywords: Disease Activity; Systemic Lupus Erythematosus; Treatment.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: DB, MC, SE, DG and DAR are employees of GSK and hold shares in the company. YT receives consulting fees, speaking fees and/or honoraria from AbbVie, Chugai, Daiichi-Sankyo, Bristol-Myers Squibb, Mitsubishi-Tanabe, Astellas, Takeda, Pfizer, Asahi-Kasei, YL Biologics, Sanofi, Janssen, Eli Lilly and GSK and has received research grants from Mitsubishi-Tanabe, Takeda, Daiichi-Sankyo, Chugai, Bristol-Myers Squibb, MSD, Astellas, AbbVie and Eisai.
Figures

References
-
- Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. 10.1136/annrheumdis-2013-205171 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

