Medication Therapy Management after Hospitalization in CKD: A Randomized Clinical Trial
- PMID: 29295829
- PMCID: PMC5967429
- DOI: 10.2215/CJN.06790617
Medication Therapy Management after Hospitalization in CKD: A Randomized Clinical Trial
Abstract
Background and objectives: CKD is characterized by remarkably high hospitalization and readmission rates. Our study aim was to test a medication therapy management intervention to reduce subsequent acute care utilization.
Design, setting, participants, & measurements: The CKD Medication Intervention Trial was a single-blind (investigators), randomized clinical trial conducted at Providence Health Care in Spokane, Washington. Patients with CKD stages 3-5 not treated by dialysis who were hospitalized for acute illness were recruited. The intervention was designed to improve posthospitalization care by medication therapy management. A pharmacist delivered the intervention as a single home visit within 7 days of discharge. The intervention included these fundamental elements: comprehensive medication review, medication action plan, and a personal medication list. The primary outcome was a composite of acute care utilization (hospital readmissions and emergency department and urgent care visits) for 90 days after hospitalization.
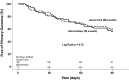
Results: Baseline characteristics of participants (n=141) included the following: age, 69±11 (mean±SD) years old; women, 48% (67 of 141); diabetes, 56% (79 of 141); hypertension, 83% (117 of 141); eGFR, 41±14 ml/min per 1.73 m2 (serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation); and urine albumin-to-creatinine ratio median, 43 mg/g (interquartile range, 8-528) creatinine. The most common primary diagnoses for hospitalization were the following: cardiovascular events, 36% (51 of 141); infections, 18% (26 of 141); and kidney diseases, 12% (17 of 141). The primary outcome occurred in 32 of 72 (44%) of the medication intervention group and 28 of 69 (41%) of those in usual care (log rank P=0.72). For only hospital readmission, the rate was 19 of 72 (26%) in the medication intervention group and 18 of 69 (26%) in the usual care group (log rank P=0.95). There was no between-group difference in achievement of guideline-based goals for use of renin-angiotensin system inhibition or for BP, hemoglobin, phosphorus, or parathyroid hormone.
Conclusions: Acute care utilization after hospitalization was not reduced by a pharmacist-led medication therapy management intervention at the transition from hospital to home.
Keywords: Acute illness; Chronic; Hospital readmission; Humans; Medication Adherence; Medication Therapy Management; Medication management; Renal Insufficiency; Transitional care; chronic kidney disease; hospitalization.
Copyright © 2018 by the American Society of Nephrology.
Figures


Comment in
-
Why Nomenclature for Pharmacist-Led Interventions Matters: Conquering the State of Confusion.Clin J Am Soc Nephrol. 2018 Feb 7;13(2):198-200. doi: 10.2215/CJN.13601217. Epub 2018 Jan 2. Clin J Am Soc Nephrol. 2018. PMID: 29295828 Free PMC article. No abstract available.
References
-
- United States Renal Data System : 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016
-
- Daratha KB, Short RA, Corbett CF, Ring ME, Alicic R, Choka R, Tuttle KR: Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol 7: 409–416, 2012 - PubMed
-
- Climente-Martí M, García-Mañón ER, Artero-Mora A, Jiménez-Torres NV: Potential risk of medication discrepancies and reconciliation errors at admission and discharge from an inpatient medical service. Ann Pharmacother 44: 1747–1754, 2010 - PubMed
-
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW: The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 138: 161–167, 2003 - PubMed
-
- Coleman EA, Smith JD, Raha D, Min SJ: Posthospital medication discrepancies: Prevalence and contributing factors. Arch Intern Med 165: 1842–1847, 2005 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

