Fibrotic Venous Remodeling and Nonmaturation of Arteriovenous Fistulas
- PMID: 29295872
- PMCID: PMC5827597
- DOI: 10.1681/ASN.2017050559
Fibrotic Venous Remodeling and Nonmaturation of Arteriovenous Fistulas
Abstract
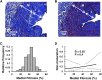
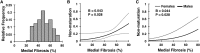
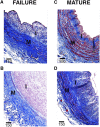
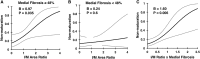
The frequency of primary failure in arteriovenous fistulas (AVFs) remains unacceptably high. This lack of improvement is due in part to a poor understanding of the pathobiology underlying AVF nonmaturation. This observational study quantified the progression of three vascular features, medial fibrosis, intimal hyperplasia (IH), and collagen fiber organization, during early AVF remodeling and evaluated the associations thereof with AVF nonmaturation. We obtained venous samples from patients undergoing two-stage upper-arm AVF surgeries at a single center, including intraoperative veins at the first-stage access creation surgery and AVFs at the second-stage transposition procedure. Paired venous samples from both stages were used to evaluate change in these vascular features after anastomosis. Anatomic nonmaturation (AVF diameter never ≥6 mm) occurred in 39 of 161 (24%) patients. Neither preexisting fibrosis nor IH predicted AVF outcomes. Postoperative medial fibrosis associated with nonmaturation (odds ratio [OR], 1.55; 95% confidence interval [95% CI], 1.05 to 2.30; P=0.03, per 10% absolute increase in fibrosis), whereas postoperative IH only associated with failure in those individuals with medial fibrosis over the population's median value (OR, 2.63; 95% CI, 1.07 to 6.46; P=0.04, per increase of 1 in the intima/media ratio). Analysis of postoperative medial collagen organization revealed that circumferential alignment of fibers around the lumen associated with AVF nonmaturation (OR, 1.38; 95% CI, 1.03 to 1.84; P=0.03, per 10° increase in angle). This study demonstrates that excessive fibrotic remodeling of the vein after AVF creation is an important risk factor for nonmaturation and that high medial fibrosis determines the stenotic potential of IH.
Keywords: arteriovenous fistula; fibrosis; vascular access.
Copyright © 2018 by the American Society of Nephrology.
Figures



References
-
- Allon M, Greene T, Dember LM, Vita JA, Cheung AK, Hamburg NM, Imrey PB, Kaufman JS, Robbin ML, Shiu YT, Terry CM, Umphrey HR, Feldman HI; Hemodialysis Fistula Maturation Study Group : Association between preoperative vascular function and postoperative arteriovenous fistula development. J Am Soc Nephrol 27: 3788–3795, 2016 - PMC - PubMed
-
- Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S: p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res 97: 533–540, 2005 - PubMed
-
- Haas TL, Doyle JL, Distasi MR, Norton LE, Sheridan KM, Unthank JL: Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol 293: H2429–H2437, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

