The Actin Cytoskeleton and Actin-Based Motility
- PMID: 29295889
- PMCID: PMC5749151
- DOI: 10.1101/cshperspect.a018267
The Actin Cytoskeleton and Actin-Based Motility
Abstract
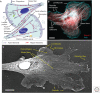
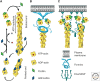
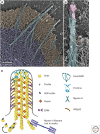
The actin cytoskeleton-a collection of actin filaments with their accessory and regulatory proteins-is the primary force-generating machinery in the cell. It can produce pushing (protrusive) forces through coordinated polymerization of multiple actin filaments or pulling (contractile) forces through sliding actin filaments along bipolar filaments of myosin II. Both force types are particularly important for whole-cell migration, but they also define and change the cell shape and mechanical properties of the cell surface, drive the intracellular motility and morphogenesis of membrane organelles, and allow cells to form adhesions with each other and with the extracellular matrix.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
