TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis
- PMID: 29295985
- PMCID: PMC5750213
- DOI: 10.1038/s41467-017-02466-4
TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis
Abstract
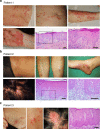
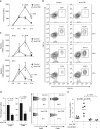
Although anti-tumor necrosis factor (TNF) agents are highly effective in the treatment of psoriasis, 2-5% of treated patients develop psoriasis-like skin lesions called paradoxical psoriasis. The pathogenesis of this side effect and its distinction from classical psoriasis remain unknown. Here we show that skin lesions from patients with paradoxical psoriasis are characterized by a selective overexpression of type I interferons, dermal accumulation of plasmacytoid dendritic cells (pDC), and reduced T-cell numbers, when compared to classical psoriasis. Anti-TNF treatment prolongs type I interferon production by pDCs through inhibition of their maturation. The resulting type I interferon overexpression is responsible for the skin phenotype of paradoxical psoriasis, which, unlike classical psoriasis, is independent of T cells. These findings indicate that paradoxical psoriasis represents an ongoing overactive innate inflammatory process, driven by pDC-derived type I interferon that does not lead to T-cell autoimmunity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures