PD-1 blockade enhances elotuzumab efficacy in mouse tumor models
- PMID: 29296719
- PMCID: PMC5728054
- DOI: 10.1182/bloodadvances.2017004382
PD-1 blockade enhances elotuzumab efficacy in mouse tumor models
Abstract
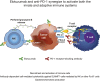
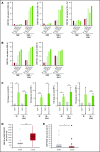
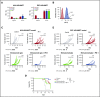
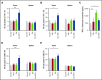
Elotuzumab, a humanized monoclonal antibody that binds human signaling lymphocytic activation molecule F7 (hSLAMF7) on myeloma cells, was developed to treat patients with multiple myeloma (MM). Elotuzumab has a dual mechanism of action that includes the direct activation of natural killer (NK) cells and the induction of NK cell-mediated antibody-dependent cellular cytotoxicity. This study aimed to characterize the effects of elotuzumab on NK cells in vitro and in patients with MM and to determine whether elotuzumab antitumor activity was improved by programmed death receptor-1 (PD-1) blockade. Elotuzumab promoted NK cell activation when added to a coculture of human NK cells and SLAMF7-expressing myeloma cells. An increased frequency of activated NK cells was observed in bone marrow aspirates from elotuzumab-treated patients. In mouse tumor models expressing hSLAMF7, maximal antitumor efficacy of a murine immunoglobulin G2a version of elotuzumab (elotuzumab-g2a) required both Fcγ receptor-expressing NK cells and CD8+ T cells and was significantly enhanced by coadministration of anti-PD-1 antibody. In these mouse models, elotuzumab-g2a and anti-PD-1 combination treatment promoted tumor-infiltrating NK and CD8+ T-cell activation, as well as increased intratumoral cytokine and chemokine release. These observations support the rationale for clinical investigation of elotuzumab/anti-PD-1 combination therapy in patients with MM.
Conflict of interest statement
Presented in oral form at the 21st annual conference of the European Hematology Association, Copenhagen, Denmark, 9-12 June 2016.Conflict-of-interest disclosure: N.A.B., K.H., A.J.K., M.D.R., and R.F.G. are employees of and/or have ownership interest in Bristol-Myers Squibb. A.J. and A.Y.K. are employees of Bristol-Myers Squibb. T.B. and M.M. were employees of Bristol-Myers Squibb at the time of the study. P.J.N. received funding from Bristol-Myers Squibb to perform aspects of this study. The remaining authors declare no competing financial interests.
Figures


References
-
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443-446. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

