Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization
- PMID: 29296728
- PMCID: PMC5727809
- DOI: 10.1182/bloodadvances.2017004390
Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization
Abstract
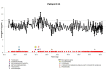
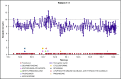
Cold agglutinin disease (CAD), a rare disease and subtype of autoimmune hemolytic anemia, is characterized by autoantibodies that bind to red blood cells at low temperatures. There is no established standard of care for CAD treatment and CAD cohort studies are limited by the rarity of the condition. The objectives of this study are to present the longitudinal experience of a CAD cohort from the United States, with a focus on anemia severity, use of medications and transfusions, and health care resource utilization. The Stanford Translational Research Integrated Database Environment database was used to retrospectively identify CAD patients diagnosed and treated at Stanford Health Care from 2000 to 2016. Twenty-nine patients were included in this analysis. There were 7.1 severe anemia events per patient-year observed over the follow-up time. For CAD patients treated at Stanford, there was a mean of 3.5 therapies per patient. Transfusions were given in at least 65% of the cohort with a mean of 11 transfusions per patient-year. For CAD-related health care use in the first year after disease onset, 93% used outpatient services with a median of 26 outpatient visits per patient. The data presented here likely represent the minimum number of events for these patients during this timeframe, as this single-center experience does not capture care from other providers. This longitudinal study of CAD patients demonstrates the severity of anemia and relapsing nature of the disease, even after administration of multiple therapies and transfusions.
Conflict of interest statement
Conflict-of-interest disclosure: M.M., X.J., L.C.B., J.P.F., H.R., E.C.C., and S.K. received a grant from True North Therapeutics, Inc for this project. A.R. is employed by True North Therapeutics, Inc and has ownership interests in a start-up company, the stock of which is not publicly traded.
Figures



References
-
- Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4(6):607-618. - PubMed
-
- Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69(4):258-271. - PubMed
-
- Genty I, Michel M, Hermine O, Schaeffer A, Godeau B, Rochant H. Caractéristiques des anémies hémolytiques auto-immunes de l’adulte: analyse rétrospective d’une série de 83 patients. Rev Med Interne. 2002;23(11):901-909. - PubMed
-
- Michel M. Cold Agglutinin Disease. Paris, France: Orphanet; 2010.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

