A phase 2 study of panobinostat with lenalidomide and weekly dexamethasone in myeloma
- PMID: 29296798
- PMCID: PMC5728465
- DOI: 10.1182/bloodadvances.2017007427
A phase 2 study of panobinostat with lenalidomide and weekly dexamethasone in myeloma
Abstract
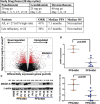
Phase 3 studies combining histone deacetylase inhibitors with bortezomib were hampered by gastrointestinal (GI) intolerance, which was not observed when combined with immunomodulatory drugs. This study is a single-center phase 2 study of panobinostat with lenalidomide and dexamethasone (FRD). Twenty-seven relapsed multiple myeloma patients were enrolled. Twenty-two patients (81%) were lenalidomide refractory and 9 (33%), 14 (52%), and 7 (26%) were refractory to pomalidomide, bortezomib, and carfilzomib, respectively. High-risk molecular findings were present in 17 (63%) patients. Responses included 2 complete responses (CRs), 4 very good partial responses (VGPRs), 5 partial responses (PRs), and 9 minimal responses (MRs) for an overall response rate of 41%, clinical benefit rate of 74%, and a disease control rate of 96%. The median progression-free survival (PFS) was 7.1 months. In the 22 lenalidomide-refractory patients, there were 1 CR, 4 VGPRs, 3 PRs, and 7 MRs, with a median PFS of 6.5 months. Median overall survival was not reached. Grade 3/4 toxicities were primarily hematologic. Gene expression profiling of enrollment tumor samples revealed a set of 1989 genes associated with short (<90 days) PFS to therapy. MAGEA1 RNA and protein expression were correlated with short PFS, and laboratory studies demonstrated a role for MAGE-A in resistance to panobinostat-induced cell death. FRD demonstrates durable responses, even in high-risk, lenalidomide-refractory patients, indicating the essential role of panobinostat in attaining responses. MAGEA1 expression may represent a functional biomarker for resistance to panobinostat. In contrast to PANORAMA 1, there were no significant GI toxicities and primarily expected hematologic toxicities. This trial was registered at www.clinicaltrials.gov as #NCT00742027.
Conflict of interest statement
Conflict-of-interest disclosure: The following authors have served as paid consultants for the identified companies: A.C. for Novartis and Celgene; S.J. for Novartis and Celgene; and D.C. and D.V. for Celgene speakers bureau. The remaining authors declare no competing financial interests.
Figures




References
-
- San-Miguel JF, Hungria VTM, Yoon SS, et al. . Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195-1206. - PubMed
-
- Richardson PG, Schlossman RL, Alsina M, et al. . PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122(14):2331-2337. - PubMed
-
- Mateos M, Spencer A, Taylor K, et al. . Phase Ib study of oral panobinostat (LBH589) plus lenalidomide (LEN) plus dexamethasone (DEX) in patients (Pts) with relapsed (Rel) or Rel and refractory (Ref) multiple myeloma (MM). J Clin Oncol. 2010;28(15 suppl):8030.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

