Gene editing rescue of a novel MPL mutant associated with congenital amegakaryocytic thrombocytopenia
- PMID: 29296828
- PMCID: PMC5728092
- DOI: 10.1182/bloodadvances.2016002915
Gene editing rescue of a novel MPL mutant associated with congenital amegakaryocytic thrombocytopenia
Abstract
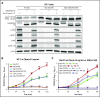
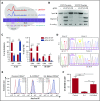
Thrombopoietin (Tpo) and its receptor (Mpl) are the principal regulators of early and late thrombopoiesis and hematopoietic stem cell maintenance. Mutations in MPL can drastically impair its function and be a contributing factor in multiple hematologic malignancies, including congenital amegakaryocytic thrombocytopenia (CAMT). CAMT is characterized by severe thrombocytopenia at birth, which progresses to bone marrow failure and pancytopenia. Here we report unique familial cases of CAMT that presented with a previously unreported MPL mutation: T814C (W272R) in the background of the activating MPL G117T (K39N or Baltimore) mutation. Confocal microscopy, proliferation and surface biotinylation assays, co-immunoprecipitation, and western blotting analysis were used to elucidate the function and trafficking of Mpl mutants. Results showed that Mpl protein bearing the W272R mutation, alone or together with the K39N mutation, lacks detectable surface expression while being strongly colocalized with the endoplasmic reticulum (ER) marker calreticulin. Both WT and K39N-mutated Mpl were found to be signaling competent, but single or double mutants bearing W272R were unresponsive to Tpo. Function of the deficient Mpl receptor could be rescued by using 2 separate approaches: (1) GRASP55 overexpression, which partially restored Tpo-induced signaling of mutant Mpl by activating an autophagy-dependent secretory pathway and thus forcing ER-trapped immature receptors to traffic to the cell surface; and (2) CRISPR-Cas9 gene editing used to repair MPL T814C mutation in transfected cell lines and primary umbilical cord blood-derived CD34+ cells. We demonstrate proof of principle for rescue of mutant Mpl function by using gene editing of primary hematopoietic stem cells, which indicates direct therapeutic applications for CAMT patients.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
-
- Tijssen MR, di Summa F, van den Oudenrijn S, et al. Functional analysis of single amino-acid mutations in the thrombopoietin-receptor Mpl underlying congenital amegakaryocytic thrombocytopenia. Br J Haematol. 2008;141(6):808-813. - PubMed
-
- Varghese LN, Zhang JG, Young SN, et al. Functional characterization of c-Mpl ectodomain mutations that underlie congenital amegakaryocytic thrombocytopenia. Growth Factors. 2014;32(1):18-26. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

