Long-term safety and efficacy of emicizumab in a phase 1/2 study in patients with hemophilia A with or without inhibitors
- PMID: 29296836
- PMCID: PMC5728143
- DOI: 10.1182/bloodadvances.2017006684
Long-term safety and efficacy of emicizumab in a phase 1/2 study in patients with hemophilia A with or without inhibitors
Abstract
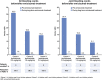
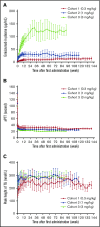
Emicizumab (ACE910), a recombinant humanized bispecific monoclonal antibody, provides factor VIII (FVIII) cofactor bridging function to restore hemostasis in people with hemophilia A. In a phase 1 trial involving 18 Japanese patients with severe hemophilia A, once-weekly subcutaneous administration of emicizumab 0.3, 1, or 3 mg/kg (cohorts 1, 2, and 3, respectively) was well tolerated and substantially reduced annualized bleeding rates (ABRs) in the presence or absence of FVIII inhibitors. The current study represents an open-label, long-term extension of the previously reported 12-week phase 1 study, in which 16 of 18 patients continued to receive emicizumab for up to 33.3 months. Long-term emicizumab treatment was well tolerated, with no thromboembolic events reported and no neutralizing antiemicizumab antibodies developing during the course of the study. Plasma concentrations of emicizumab increased in a dose-proportional manner, with activated partial thromboplastin times remaining short. In cohorts 1, 2, and 3, respectively, median ABRs remained low at 1.4, 0.2, and 0 compared with 4.4, 0, and 0 in the 12-week study. Overall, 8 patients experienced no bleeding events (6 patients with and 2 patients without FVIII inhibitors); dose up-titration resulted in further reduction in ABRs in patients with suboptimal bleeding control; and the episodic use of clotting factors to control bleeding was reduced. In conclusion, long-term emicizumab treatment demonstrated a favorable safety profile with encouraging efficacy, irrespective of the presence of FVIII inhibitors, in patients with hemophilia A. This study was registered at www.clinicaltrials.jp as #JapicCTI-132195.
Conflict of interest statement
Conflict-of-interest disclosure: This work was supported by Chugai Pharmaceutical Co., Ltd (honoraria [M.S., K.N.], board of directors or advisory committee membership [M.S., M.T., T.M., K.F.]; research funding [M.S., H.H., M.T., T.M., T.S., K.F., K.N.]; employment [R.K., K.Y., H.Y.]), and F. Hoffmann-La Roche Ltd (consultancy [M.S.]); M.S., K.Y., and K.N. are listed on patents for anti-FIXa/X bispecific antibodies.
Figures


References
-
- Mannucci PM, Tuddenham EG. The hemophilias--from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773-1779. - PubMed
-
- Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361(9371):1801-1809. - PubMed
-
- Tsailas PG, Wiedel JD. Arthrodesis of the ankle and subtalar joints in patients with haemophilic arthropathy. Haemophilia. 2010;16(5):822-831. - PubMed
-
- Luck JV Jr, Silva M, Rodriguez-Merchan EC, Ghalambor N, Zahiri CA, Finn RS. Hemophilic arthropathy. J Am Acad Orthop Surg. 2004;12(4):234-245. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

