Pak2 regulates myeloid-derived suppressor cell development in mice
- PMID: 29296839
- PMCID: PMC5728145
- DOI: 10.1182/bloodadvances.2017007435
Pak2 regulates myeloid-derived suppressor cell development in mice
Abstract
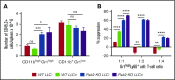
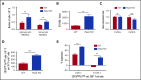
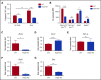
Myeloid-derived suppressor cells (MDSCs) are CD11b+Gr1+ cells that induce T-cell hyporesponsiveness, thus impairing antitumor immunity. We have previously reported that disruption of Pak2, a member of the p21-activated kinases (Paks), in hematopoietic stem/progenitor cells (HSPCs) induces myeloid lineage skewing and expansion of CD11bhighGr1high cells in mice. In this study, we confirmed that Pak2-KO CD11bhighGr1high cells suppressed T-cell proliferation, consistent with an MDSC phenotype. Loss of Pak2 function in HSPCs led to (1) increased hematopoietic progenitor cell sensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling, (2) increased MDSC proliferation, (3) decreased MDSC sensitivity to both intrinsic and Fas-Fas ligand-mediated apoptosis, and (4) promotion of MDSCs by Pak2-deficient CD4+ T cells that produced more interferon γ, tumor necrosis factor α, and GM-CSF. Pak2 disruption activated STAT5 while downregulating the expression of IRF8, a well-described myeloid transcription factor. Together, our data reveal a previously unrecognized role of Pak2 in regulating MDSC development via both cell-intrinsic and extrinsic mechanisms. Our findings have potential translational implications, as the efficacy of targeting Paks in cancer therapeutics may be undermined by tumor escape from immune control and/or acceleration of tumorigenesis through MDSC expansion.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

