Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation
- PMID: 29296895
- PMCID: PMC5729622
- DOI: 10.1182/bloodadvances.2017009845
Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation
Abstract
Nowadays, minimal residual disease (MRD) is accepted as the strongest independent prognostic factor in acute lymphoblastic leukemia (ALL). It can be detected by molecular methods that use leukemia-specific or patient-specific molecular markers (fusion gene transcripts, or immunoglobulin/T-cell receptor [IG/TR] gene rearrangements), and by multi-parametric flow cytometry. The sensitivity and specificity of these methods can vary across treatment time points and therapeutic settings. Thus, knowledge of the principles and limitations of each technology is of the utmost importance for correct interpretation of MRD results. Time will tell whether new molecular and flow cytometric high-throughput technologies can overcome the limitations of current standard methods and eventually bring additional benefits. MRD during standard ALL chemotherapy is the strongest overall prognostic indicator and has therefore been used for refining initial treatment stratification. Moreover, MRD positivity after the maintenance phase of treatment may point to an impending relapse and thus enable salvage treatment to be initiated earlier, which could possibly improve treatment results. The prognostic relevance of pretransplantation MRD was shown by several studies, and MRD high-risk patients were shown to benefit from stem cell transplantation (SCT). Also, MRD positivity after SCT correlates with worse outcomes. In addition, MRD information is very instructive in current clinical trials that test novel agents to evaluate their treatment efficacy. Although conventional clinical risk factors lose their independent prognostic significance when combined with MRD information, recently identified genetic markers may further improve the treatment stratification in ALL.
Conflict of interest statement
Conflict-of-interest disclosure: M.B. received honoraria from Amgen, Inc. and Roche Pharma AG; received financial support for reference diagnostics from Amgen, Inc., Affimed, and Regeneron; was a member of the Speakers Bureau for Amgen, Inc., and Pfizer Oncology; received research funding from Amgen, Inc.; and served as a consultant for Incyte. M.K. declares no competing financial interests.
Figures

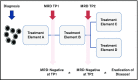
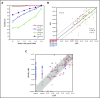
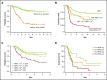
References
-
- Fielding AK, Richards SM, Chopra R, et al. ; Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944-950. - PubMed
-
- Gökbuget N, Stanze D, Beck J, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032-2041. - PubMed
-
- Kotrova M, van der Velden VH, van Dongen JJ, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant. 2017;52(7):962-968. - PubMed
-
- Fronkova E, Muzikova K, Mejstrikova E, et al. B-cell reconstitution after allogeneic SCT impairs minimal residual disease monitoring in children with ALL. Bone Marrow Transplant. 2008;42(3):187-196. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

