TLR2 agonism reverses chemotherapy-induced neutropenia in Macaca fascicularis
- PMID: 29296907
- PMCID: PMC5728636
- DOI: 10.1182/bloodadvances.2017010611
TLR2 agonism reverses chemotherapy-induced neutropenia in Macaca fascicularis
Abstract
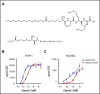
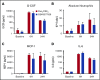
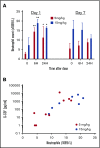
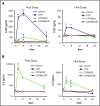
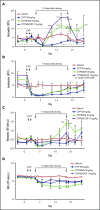
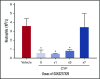
Neutropenia is a common consequence of radiation and chemotherapy in cancer patients. The resulting immunocompromised patients become highly susceptible to potentially life-threatening infections. Granulocyte colony-stimulating factor (G-CSF) is known to stimulate neutrophil production and is widely used as a treatment of chemotherapy-induced neutropenia. A small-molecule G-CSF secretagogue without a requirement for refrigerated supply chain would offer a more convenient and cost-effective treatment of chemotherapy-induced neutropenia. Bacterial lipopeptides activate innate immune responses through Toll-like receptor 2 (TLR2) and induce the release of cytokines, including G-CSF, from macrophages, monocytes, and endothelial. Pam2CSK4 is a synthetic lipopeptide that effectively mimics bacterial lipoproteins known to activate TLR2 receptor signaling through the TLR2/6 heterodimer. Substrate-based drug design led to the discovery of GSK3277329, which stimulated the release of G-CSF in activated THP-1 cells, peripheral blood mononuclear cells, and human umbilical vein endothelial cells. When administered subcutaneously to cynomolgus monkeys (Macaca fascicularis), GSK3277329 caused systemic elevation of G-CSF and interleukin-6 (IL-6), but not IL-1β or tumor necrosis factor α, indicating a selective cytokine-stimulation profile. Repeat daily injections of GSK3277329 in healthy monkeys also raised circulating neutrophils above the normal range over a 1-week treatment period. More importantly, repeated daily injections of GSK3277329 over a 2-week period restored neutrophil loss in monkeys given chemotherapy treatment (cyclophosphamide, Cytoxan). These data demonstrate preclinical in vivo proof of concept that TLR2 agonism can drive both G-CSF induction and subsequent neutrophil elevation in the cynomolgus monkey and could be a therapeutic strategy for the treatment of chemotherapy-induced neutropenia.
Conflict of interest statement
Conflict-of-interest disclosure: The authors are employees of GlaxoSmithKline PLC.
Figures

References
-
- Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. - PubMed
-
- Nagata S, Tsuchiya M, Asano S, et al. . Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature. 1986;319(6052):415-418. - PubMed
-
- Souza LM, Boone TC, Gabrilove J, et al. . Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232(4746):61-65. - PubMed
-
- Morstyn G, Campbell L, Lieschke G, et al. . Treatment of chemotherapy-induced neutropenia by subcutaneously administered granulocyte colony-stimulating factor with optimization of dose and duration of therapy. J Clin Oncol. 1989;7(10):1554-1562. - PubMed
-
- Sheridan WP, Begley CG, Juttner CA, et al. . Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339(8794):640-644. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

