Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib
- PMID: 29296914
- PMCID: PMC5728643
- DOI: 10.1182/bloodadvances.2017011999
Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib
Erratum in
-
Bye AP, Unsworth AJ, Desborough MJ, et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1(26):2610-2623.Blood Adv. 2018 Dec 11;2(23):3515. doi: 10.1182/bloodadvances.2018028944. Blood Adv. 2018. PMID: 30530778 Free PMC article. No abstract available.
Abstract
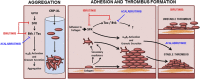
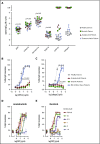
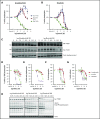
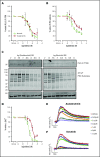
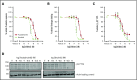
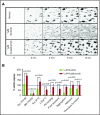
The Bruton tyrosine kinase (Btk) inhibitor ibrutinib induces platelet dysfunction and causes increased risk of bleeding. Off-target inhibition of Tec is believed to contribute to platelet dysfunction and other side effects of ibrutinib. The second-generation Btk inhibitor acalabrutinib was developed with improved specificity for Btk over Tec. We investigated platelet function in patients with non-Hodgkin lymphoma (NHL) receiving ibrutinib or acalabrutinib by aggregometry and by measuring thrombus formation on collagen under arterial shear. Both patient groups had similarly dysfunctional aggregation responses to collagen and collagen-related peptide, and comparison with mechanistic experiments in which platelets from healthy donors were treated with the Btk inhibitors suggested that both drugs inhibit platelet Btk and Tec at physiological concentrations. Only ibrutinib caused dysfunctional thrombus formation, whereas size and morphology of thrombi following acalabrutinib treatment were of normal size and morphology. We found that ibrutinib but not acalabrutinib inhibited Src family kinases, which have a critical role in platelet adhesion to collagen that is likely to underpin unstable thrombus formation observed in ibrutinib patients. We found that platelet function was enhanced by increasing levels of von Willebrand factor (VWF) and factor VIII (FVIII) ex vivo by addition of intermediate purity FVIII (Haemate P) to blood from patients, resulting in consistently larger thrombi. We conclude that acalabrutinib avoids major platelet dysfunction associated with ibrutinib therapy, and platelet function may be enhanced in patients with B-cell NHL by increasing plasma VWF and FVIII.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Futatani T, Watanabe C, Baba Y, Tsukada S, Ochs HD. Bruton’s tyrosine kinase is present in normal platelets and its absence identifies patients with X-linked agammaglobulinaemia and carrier females. Br J Haematol. 2001;114(1):141-149. - PubMed
-
- Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 2003;102(10):3592-3599. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

