Isolation, Derivative Synthesis, and Structure-Activity Relationships of Antiparasitic Bromopyrrole Alkaloids from the Marine Sponge Tedania brasiliensis
- PMID: 29297684
- PMCID: PMC5989537
- DOI: 10.1021/acs.jnatprod.7b00876
Isolation, Derivative Synthesis, and Structure-Activity Relationships of Antiparasitic Bromopyrrole Alkaloids from the Marine Sponge Tedania brasiliensis
Abstract
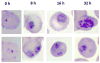
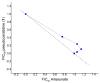
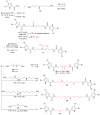
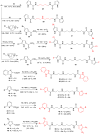
The isolation and identification of a series of new pseudoceratidine (1) derivatives from the sponge Tedania brasiliensis enabled the evaluation of their antiparasitic activity against Plasmodium falciparum, Leishmania (Leishmania) amazonensis, Leishmania (Leishmania) infantum, and Trypanosoma cruzi, the causative agents of malaria, cutaneous leishmaniasis, visceral leishmaniasis, and Chagas disease, respectively. The new 3-debromopseudoceratidine (4), 20-debromopseudoceratidine (5), 4-bromopseudoceratidine (6), 19-bromopseudoceratidine (7), and 4,19-dibromopseudoceratidine (8) are reported. New tedamides A-D (9-12), with an unprecedented 4-bromo-4-methoxy-5-oxo-4,5-dihydro-1H-pyrrole-2-carboxamide moiety, are also described. Compounds 4 and 5, 6 and 7, 9 and 10, and 11 and 12 have been isolated as pairs of inseparable structural isomers differing in their sites of bromination or oxidation. Tedamides 9+10 and 11+12 were obtained as optically active pairs, indicating an enzymatic formation rather than an artifactual origin. N12-Acetylpseudoceratidine (2) and N12-formylpseudoceratidine (3) were obtained by derivatization of pseudoceratidine (1). The antiparasitic activity of pseudoceratidine (1) led us to synthesize 23 derivatives (16, 17, 20, 21, 23, 25, 27-29, 31, 33, 35, 38, 39, 42, 43, 46, 47, 50, and 51) with variations in the polyamine chain and aromatic moiety in sufficient amounts for biological evaluation in antiparasitic assays. The measured antimalarial activity of pseudoceratidine (1) and derivatives 4, 5, 16, 23, 25, 31, and 50 provided an initial SAR evaluation of these compounds as potential leads for antiparasitics against Leishmania amastigotes and against P. falciparum. The results obtained indicate that pseudoceratidine represents a promising scaffold for the development of new antimalarial drugs.
Figures

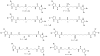



References
-
- Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Nat Prod Rep. 2017;34:235–294. and previous reviews in this series. - PubMed
-
- Braekman JC, Daloze D, Stoller C, van Soest RWM. Biochem Syst Ecol. 1992;20:417–431.
-
- van Soest RWM, Braekman JC. Mem Queensland Mus. 1999;44:569–589.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

