Hair Follicle Development in Mouse Pluripotent Stem Cell-Derived Skin Organoids
- PMID: 29298425
- PMCID: PMC5806130
- DOI: 10.1016/j.celrep.2017.12.007
Hair Follicle Development in Mouse Pluripotent Stem Cell-Derived Skin Organoids
Abstract
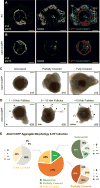
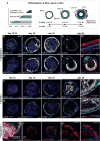
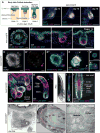
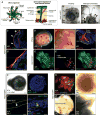
The mammalian hair follicle arises during embryonic development from coordinated interactions between the epidermis and dermis. It is currently unclear how to recapitulate hair follicle induction in pluripotent stem cell cultures for use in basic research studies or in vitro drug testing. To date, generation of hair follicles in vitro has only been possible using primary cells isolated from embryonic skin, cultured alone or in a co-culture with stem cell-derived cells, combined with in vivo transplantation. Here, we describe the derivation of skin organoids, constituting epidermal and dermal layers, from a homogeneous population of mouse pluripotent stem cells in a 3D culture. We show that skin organoids spontaneously produce de novo hair follicles in a process that mimics normal embryonic hair folliculogenesis. This in vitro model of skin development will be useful for studying mechanisms of hair follicle induction, evaluating hair growth or inhibitory drugs, and modeling skin diseases.
Keywords: 3D culture; dermis; epidermis; hair follicle; organoids; pluripotent stem cells; skin; skin appendages.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
J.L. and K.R.K, with the Indiana University Research and Technology Corporation, have applied for a patent related to the cell culture method described in this manuscript.
Figures

References
-
- Coraux C, Hilmi C, Rouleau M, Spadafora A, Hinnrasky J, Ortonne JP, Dani C, Aberdam D. Reconstituted skin from murine embryonic stem cells. Curr Biol. 2003;13:849–853. - PubMed
-
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

