The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain
- PMID: 29298427
- PMCID: PMC5783200
- DOI: 10.1016/j.celrep.2017.12.039
The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain
Abstract
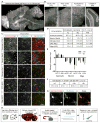
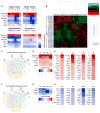
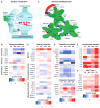
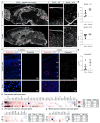
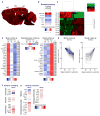
Aging brains undergo cognitive decline, associated with decreased neuronal synapse number and function and altered metabolism. Astrocytes regulate neuronal synapse formation and function in development and adulthood, but whether these properties change during aging, contributing to neuronal dysfunction, is unknown. We addressed this by generating aged and adult astrocyte transcriptomes from multiple mouse brain regions. These data provide a comprehensive RNA-seq database of adult and aged astrocyte gene expression, available online as a resource. We identify astrocyte genes altered by aging across brain regions and regionally unique aging changes. Aging astrocytes show minimal alteration of homeostatic and neurotransmission-regulating genes. However, aging astrocytes upregulate genes that eliminate synapses and partially resemble reactive astrocytes. We further identified heterogeneous expression of synapse-regulating genes between astrocytes from different cortical regions. We find that alterations to astrocytes in aging create an environment permissive to synapse elimination and neuronal damage, potentially contributing to aging-associated cognitive decline.
Keywords: aging; astrocyte; neurodegeneration; synapse.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol. 2014;30:439–463. - PubMed
-
- Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Hippocampal Astrocyte Cultures from Adult and Aged Rats Reproduce Changes in Glial Functionality Observed in the Aging Brain. Mol Neurobiol. 2017;54:2969–2985. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

