The Brainfit study: efficacy of cognitive training and exergaming in pediatric cancer survivors - a randomized controlled trial
- PMID: 29298678
- PMCID: PMC5753470
- DOI: 10.1186/s12885-017-3933-x
The Brainfit study: efficacy of cognitive training and exergaming in pediatric cancer survivors - a randomized controlled trial
Abstract
Background: Cancer survival comes at a price: pediatric cancer survivors bear a high risk for a wide range of cognitive difficulties. Therefore, interventions targeting these difficulties are required. The aim of the present clinical trial is to extend empirical evidence about efficacy of cognitive and physical training in pediatric cancer survivors. It is hypothesized that early cognitive and physical interventions affect the remediation of pediatric cancer survivors in terms of improved executive functions (primary outcome). Additional positive effects of cognitive and physical intervention to other areas such as memory and attention are expected (secondary outcome). Changes in cognitive performance are expected to be associated with structural and functional changes in the brain.
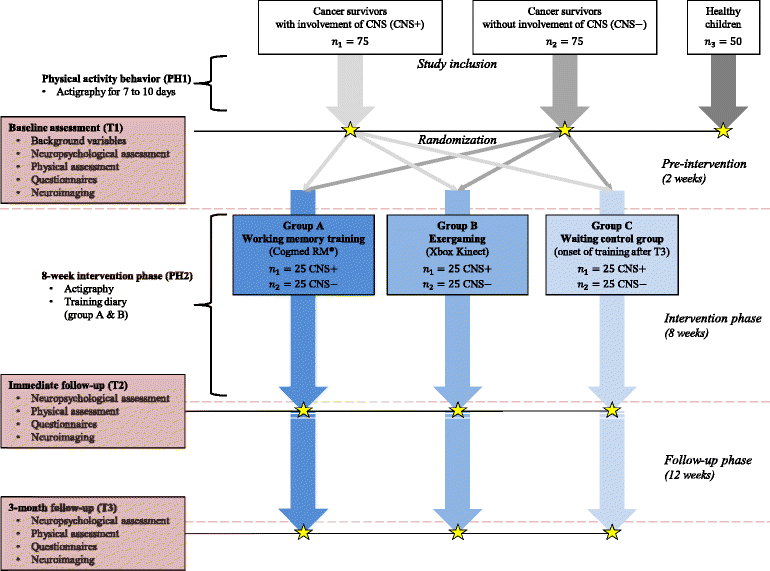
Methods: Overall, 150 pediatric cancer survivors and 50 matched controls will be included in this trial. The cancer survivors will be randomly assigned to either a computerized cognitive training, a physical training (exergaming) or a waiting control group. They will be assessed with neuropsychological tests, tests of sport motor performance and physical fitness before and after 8 weeks of training and again at a 3-months follow-up. Moreover, neuroimaging will be performed at each of the three time points to investigate the training impact on brain structure and function.
Discussion: With increasing cancer survival rates, evidence-based interventions are of particular importance. New insights into training-related plasticity in the developing brain will further help to develop tailored rehabilitation programs for pediatric cancer survivors.
Trial registration: KEK BE 196/15; KEK ZH 2015-0397; ICTRP NCT02749877 ; date of registration: 30.11.2016; date of first participant enrolment: .18.01.2017.
Keywords: Active video gaming; Brain tumor; Childhood cancer survivors; Physical exercise; Physical training; Working memory training.
Conflict of interest statement
Ethics approval and consent to participate
The submitted research project has been approved by the ethics committee of the canton of Bern, Switzerland and the canton of Zurich, Switzerland (KEK BE 196/15 (30.11.16); KEK ZH 2015–0397). Important study protocol modifications will be reported to relevant parties. In addition, the research project has been registered with the International Clinical Trials Registry Platform (ICTRP NCT02749877), which is a WHO primary registry. After verifying that participants are eligible for the study, the participants and their parents will receive adequate verbal information about the study. They will be given enough time to consider their participation and the informed consent information will be sent to them. Written informed consent will be obtained of all participants and their parents or caregivers. Participants can withdraw from participating at any time, without giving reason for it. If participants discontinue, their data will still be analyzed. Individual medical information obtained as a result of this study is considered confidential and disclosure to third parties is prohibited. Participants’ confidentiality will be further ensured by utilising subject identification code numbers (both on paper and electronically) to correspond to treatment data in the computer files. The key (i.e. a list in which an alphanumeric code is linked to individual participants’ names) will be kept separately from the study data in a secured (locked) document. Access to documents, datasets, statistical codes, etc. during and after the study will only be granted to the PI and his designees (e.g., co-authors of the study protocol). The results of the present study will be submitted for publication in peer-reviewed journals and will be presented at national and international scientific meetings to healthcare professionals and/or the public.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Xu J, Murphy SL, Kochanek KD, Birgham AB. National vital statistics reports deaths : final data for 2013. National Center for Healh. Statistics. 2012;60:1–117.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical