Effect of adoption of neoadjuvant chemotherapy for advanced ovarian cancer on all cause mortality: quasi-experimental study
- PMID: 29298771
- PMCID: PMC5751831
- DOI: 10.1136/bmj.j5463
Effect of adoption of neoadjuvant chemotherapy for advanced ovarian cancer on all cause mortality: quasi-experimental study
Abstract
Objective: To estimate the causal effect of increased use of neoadjuvant chemotherapy (NACT) on all cause mortality in advanced epithelial ovarian cancer.
Design: Quasi-experimental fuzzy regression discontinuity design and cross sectional analysis.
Setting: Cancer programs throughout the United States accredited by the Commission on Cancer.
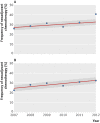
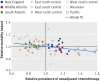
Participants: 6034 women with a diagnosis of stage 3C or 4 epithelial ovarian cancer from regions that rapidly adopted use of NACT from 2011 to 2012 (27% increase in the New England and east south central regions) or remained unchanged (control regions, south Atlantic, west north central, and east north central regions).
Main outcome measure: All cause mortality within three years of diagnosis. Kaplan-Meier curves and proportional hazard models were estimated to compare mortality differences between rapidly adopting regions and controls.
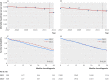
Results: 1156 women were treated for advanced epithelial ovarian cancer during 2011 and 2012 in the two rapidly adopting regions and 4878 women in the three control regions. In the rapidly adopting regions, patients treated in 2012 compared with 2011 had a mortality hazard ratio of 0.81 (95% confidence interval 0.71 to 0.94) after adjusting for mortality time trends, whereas no difference was observed in control regions (1.02, 0.93 to 1.12). Compared with control regions, larger declines in 90 day surgical mortality (7.0% to 4.0% v 5.0% to 4.3%, P=0.01) and in the proportion of women not receiving surgery and chemotherapy (20.0% to 17.4% v 19.0 to 19.5%, P=0.04) were observed in rapidly adopting regions. Cross sectional analysis confirmed that treatment in regions with greater use of NACT was associated was lower mortality (P=0.001).
Conclusions: Adoption of NACT for advanced epithelial ovarian cancer in New England and east south central regions led to a sizable reduction in mortality within three years after diagnosis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
