Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib
- PMID: 29298799
- PMCID: PMC6434713
- DOI: 10.1158/1078-0432.CCR-17-1875
Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib
Abstract
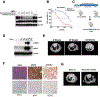
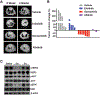
Purpose:HER2 (or ERBB2) aberrations, including both amplification and mutations, have been classified as oncogenic drivers that contribute to 2% to 6% of lung adenocarcinomas. HER2 amplification is also an important mechanism for acquired resistance to EGFR tyrosine kinase inhibitors (TKI). However, due to limited preclinical studies and clinical trials, currently there is still no available standard of care for lung cancer patients with HER2 aberrations. To fulfill the clinical need for targeting HER2 in patients with non-small cell lung cancer (NSCLC), we performed a comprehensive preclinical study to evaluate the efficacy of a third-generation TKI, osimertinib (AZD9291).Experimental Design: Three genetically modified mouse models (GEMM) mimicking individual HER2 alterations in NSCLC were generated, and osimertinib was tested for its efficacy against these HER2 aberrations in vivoResults: Osimertinib treatment showed robust efficacy in HER2wt overexpression and EGFR del19/HER2 models, but not in HER2 exon 20 insertion tumors. Interestingly, we further identified that combined treatment with osimertinib and the BET inhibitor JQ1 significantly increased the response rate in HER2-mutant NSCLC, whereas JQ1 single treatment did not show efficacy.Conclusions: Overall, our data indicated robust antitumor efficacy of osimertinib against multiple HER2 aberrations in lung cancer, either as a single agent or in combination with JQ1. Our study provides a strong rationale for future clinical trials using osimertinib either alone or in combination with epigenetic drugs to target aberrant HER2 in patients with NSCLC. Clin Cancer Res; 24(11); 2594-604. ©2018 AACRSee related commentary by Cappuzzo and Landi, p. 2470.
©2018 American Association for Cancer Research.
Figures




Comment in
-
HER2 Deregulation in Lung Cancer: Right Time to Adopt an Orphan?Clin Cancer Res. 2018 Jun 1;24(11):2470-2472. doi: 10.1158/1078-0432.CCR-18-0198. Epub 2018 Mar 1. Clin Cancer Res. 2018. PMID: 29496760
References
-
- Ke EE, Wu YL. EGFR as a pharmacological target in EGFR-mutant nonsmall-cell lung cancer: where do we stand now? Trends Pharmacol Sci 2016;37:887–903. - PubMed
-
- Thomas A, Liu SV, Subramaniam DS, Giaccone G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol 2015;12:511–26. - PubMed
-
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer 2013;13:685–700. - PubMed
-
- Liao BC, Lin CC, Yang JC. Second and third-generation epidermal growth factor receptor tyrosine kinase inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol 2015;27:94–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

