Rapid Cloning of Novel Rhesus Adenoviral Vaccine Vectors
- PMID: 29298888
- PMCID: PMC5827402
- DOI: 10.1128/JVI.01924-17
Rapid Cloning of Novel Rhesus Adenoviral Vaccine Vectors
Abstract
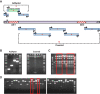
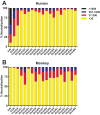
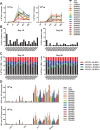
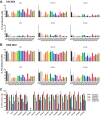
Human and chimpanzee adenovirus vectors are being developed to circumvent preexisting antibodies against common adenovirus vectors such as Ad5. However, baseline immunity to these vectors still exists in human populations. Traditional cloning of new adenovirus vaccine vectors is a long and cumbersome process that takes 2 months or more and that requires rare unique restriction enzyme sites. Here we describe a novel, restriction enzyme-independent method for rapid cloning of new adenovirus vaccine vectors that reduces the total cloning procedure to 1 week. We developed 14 novel adenovirus vectors from rhesus monkeys that can be grown to high titers and that are immunogenic in mice. All vectors grouped with the unusual adenovirus species G and show extremely low seroprevalence in humans. Rapid cloning of novel adenovirus vectors is a promising approach for the development of new vector platforms. Rhesus adenovirus vectors may prove useful for clinical development.IMPORTANCE To overcome baseline immunity to human and chimpanzee adenovirus vectors, we developed 14 novel adenovirus vectors from rhesus monkeys. These vectors are immunogenic in mice and show extremely low seroprevalence in humans. Rhesus adenovirus vectors may prove useful for clinical development.
Keywords: adenoviruses; live vector vaccines; rhesus monkey; vaccines.
Copyright © 2018 Abbink et al.
Figures

References
-
- Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, Ervin JE, Greenberg RN, Strout C, Treanor JJ, Webby R, Wright PF. 2013. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis 13:238–250. doi: 10.1016/S1473-3099(12)70345-6. - DOI - PMC - PubMed
-
- Alexander J, Mendy J, Vang L, Avanzini JB, Garduno F, Manayani DJ, Ishioka G, Farness P, Ping LH, Swanstrom R, Parks R, Liao HX, Haynes BF, Montefiori DC, LaBranche C, Smith J, Gurwith M, Mayall T. 2013. Pre-clinical development of a recombinant, replication-competent adenovirus serotype 4 vector vaccine expressing HIV-1 envelope 1086 clade C. PLoS One 8:e82380. doi: 10.1371/journal.pone.0082380. - DOI - PMC - PubMed
-
- Green CA, Scarselli E, Voysey M, Capone S, Vitelli A, Nicosia A, Cortese R, Thompson AJ, Sande CS, de Lara C, Klenerman P, Pollard AJ. 2015. Safety and immunogenicity of novel respiratory syncytial virus (RSV) vaccines based on the RSV viral proteins F, N and M2-1 encoded by simian adenovirus (PanAd3-RSV) and MVA (MVA-RSV); protocol for an open-label, dose-escalation, single-centre, phase 1 clinical trial in healthy adults. BMJ Open 5:e008748. doi: 10.1136/bmjopen-2015-008748. - DOI - PMC - PubMed
-
- Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng‘ang'a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. 2015. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. doi: 10.1126/science.aab3886. - DOI - PMC - PubMed
-
- Rampling T, Ewer KJ, Bowyer G, Bliss CM, Edwards NJ, Wright D, Payne RO, Venkatraman N, de Barra E, Snudden CM, Poulton ID, de Graaf H, Sukhtankar P, Roberts R, Ivinson K, Weltzin R, Rajkumar B-Y, Wille-Reece U, Lee CK, Ockenhouse CF, Sinden RE, Gerry S, Lawrie AM, Vekemans J, Morelle D, Lievens M, Ballou RW, Cooke GS, Faust SN, Gilbert S, Hill AVS. 2016. Safety and high level efficacy of the combination malaria vaccine regimen of RTS,S/AS01B with chimpanzee adenovirus 63 and modified vaccinia Ankara vectored vaccines expressing ME-TRAP. J Infect Dis 214:772–781. doi: 10.1093/infdis/jiw244. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

