Design strategies for dynamic closed-loop optogenetic neurocontrol in vivo
- PMID: 29300002
- PMCID: PMC5957547
- DOI: 10.1088/1741-2552/aaa506
Design strategies for dynamic closed-loop optogenetic neurocontrol in vivo
Abstract
Objective: Controlling neural activity enables the possibility of manipulating sensory perception, cognitive processes, and body movement, in addition to providing a powerful framework for functionally disentangling the neural circuits that underlie these complex phenomena. Over the last decade, optogenetic stimulation has become an increasingly important and powerful tool for understanding neural circuit function, owing to the ability to target specific cell types and bidirectionally modulate neural activity. To date, most stimulation has been provided in open-loop or in an on/off closed-loop fashion, where previously-determined stimulation is triggered by an event. Here, we describe and demonstrate a design approach for precise optogenetic control of neuronal firing rate modulation using feedback to guide stimulation continuously.
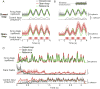
Approach: Using the rodent somatosensory thalamus as an experimental testbed for realizing desired time-varying patterns of firing rate modulation, we utilized a moving average exponential filter to estimate firing rate online from single-unit spiking measured extracellularly. This estimate of instantaneous rate served as feedback for a proportional integral (PI) controller, which was designed during the experiment based on a linear-nonlinear Poisson (LNP) model of the neuronal response to light.
Main results: The LNP model fit during the experiment enabled robust closed-loop control, resulting in good tracking of sinusoidal and non-sinusoidal targets, and rejection of unmeasured disturbances. Closed-loop control also enabled manipulation of trial-to-trial variability.
Significance: Because neuroscientists are faced with the challenge of dissecting the functions of circuit components, the ability to maintain control of a region of interest in spite of changes in ongoing neural activity will be important for disambiguating function within networks. Closed-loop stimulation strategies are ideal for control that is robust to such changes, and the employment of continuous feedback to adjust stimulation in real-time can improve the quality of data collected using optogenetic manipulation.
Figures







References
-
- Madisen Linda, Mao Tianyi, Koch Henner, Zhuo Jia-min, Berenyi Antal, Fujisawa Shigeyoshi, Hsu Yun-Wei A, Garcia Alfredo J, III, Gu Xuan, Zanella Sebastien, et al. A toolbox of cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature Neuroscience. 2012;15(5):793–802. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
