Long-term Nonhuman Primate Renal Allograft Survival Without Ongoing Immunosuppression in Recipients of Delayed Donor Bone Marrow Transplantation
- PMID: 29300231
- PMCID: PMC5860973
- DOI: 10.1097/TP.0000000000002078
Long-term Nonhuman Primate Renal Allograft Survival Without Ongoing Immunosuppression in Recipients of Delayed Donor Bone Marrow Transplantation
Abstract
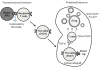
Background: We have previously reported successful induction of renal allograft tolerance in nonhuman primates (NHP) after an initial posttransplant period of conventional immunosuppression (delayed tolerance) using a nonmyeloablative conditioning regimen consisting of anti-CD154 and anti-CD8 mAbs plus equine antithymocyte globulin (Atgam) and donor bone marrow transplantation (DBMT). Because these reagents are not currently clinically available, the protocol was revised to be applicable to human recipients of deceased donor allografts.
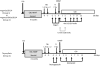
Method: Four cynomolgus monkeys received major histocompatibility complex-mismatched kidney allografts with conventional immunosuppression for 4 months. The recipients were then treated with a nonmyeloablative conditioning regimen consisting of thymoglobulin, belatacept, and DBMT. The results were compared with recipients treated with conditioning regimen consisting of Atgam and anti-CD154 mAb, with and without anti-CD8 mAb.
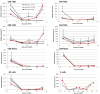
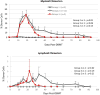
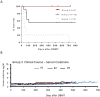
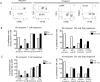
Results: In 4 consecutive NHP recipients treated with the modified conditioning regimen, homeostatic recovery of CD8 TEM was delayed until after day 20 and multilineage chimerism was successfully induced. Three of the 4 recipients achieved long-term allograft survival (>728, >540, >449 days) without ongoing maintenance immunosuppression. Posttransplant MLR showed loss of antidonor CD8 T cell and CD4 IFNγ responses with expansion of CD4FOXP3 regulatory T cells. However, the late development of donor-specific antibody in NHP recipients confirms the need for additional anti-B-cell depletion with agents, such as rituximab, as has been shown in our clinical trials.
Conclusions: This study provides proof of principle that induction of mixed chimerism and long-term renal allograft survival without immunosuppression after delayed DBMT is possible with clinically available reagents.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–262. - PubMed
-
- Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4(9):1391–1398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

